Waseda Frontline Research Feature Vol. 19: Exploring Undiscovered Potential of Catalysts (Part 2 of 4)
Mon, Apr 16, 2018-
Tags
Yasushi Sekine
Professor, Department of Applied Chemistry, School of Advanced Science and Engineering, Faculty of Science and Engineering
Research Field: Catalytic Chemistry
Part 2: Capturing Electrocatalytic Reactions Live
 Catalytic reactions are dramatically accelerated by applying an electric field, a space acted upon by a weak electrical force. Professor Yasushi Sekine discovered such electrocatalytic reactions, which had been a completely unknown phenomenon. He then began to try and explain the mechanism of electrocatalytic reactions, and his strategy was to capture the undergoing reactions live. (Interviewed on September 7, 2017)
Catalytic reactions are dramatically accelerated by applying an electric field, a space acted upon by a weak electrical force. Professor Yasushi Sekine discovered such electrocatalytic reactions, which had been a completely unknown phenomenon. He then began to try and explain the mechanism of electrocatalytic reactions, and his strategy was to capture the undergoing reactions live. (Interviewed on September 7, 2017)
Mechanism of Electrocatalytic Reactions
As I mentioned in Part 1, I discovered in 2009 that when a weak electric field was applied to a catalytic reaction to obtain hydrogen, the reaction proceeded satisfactorily even at a low temperature of 150°C. I called this an electrocatalytic reaction, which was a major discovery because previous methods required high temperatures of 700°C or more.
However, the mechanism of electrocatalytic reactions remained a mystery. Why did applying a weak electric field accelerate catalytic reactions? In my research, I have aimed to produce results that would be beneficial for society. Even though I had identified this phenomenon, I needed to understand its mechanism in order to make it even more efficient and apply it to other catalytic reasons. Clarifying the mechanism of electrocatalytic reactions became my next big research goal.
Monitoring Fish without Harming Them
To unveil the mechanism of electrocatalytic reactions, we also looked at a hot technology in the field of analytical techniques called operando spectroscopy. Operando is Latin for “working,” and in this method, we monitor the exact moment when the catalytic reaction actually proceeds.
Let me explain operando spectroscopy with an analogy. For instance, if we wanted to find out how fish breathes, we would have to slice a fish into three strips on a cutting board, look at its gills, and learn that the gills are where fish obtain oxygen. In this method, we would have to kill the fish before examining it. Preferably, it would be better to understand how fish breathe by investigating a live fish. So, we could instead employ a method of placing the fish in a fish tank and fitting its body with a sensor that can detect how it breathes, allowing us to obtain data while keeping it alive. This method would also allow us to observe its actual bodily movement, but fitting a sensor to the body of a fish in a small fish tank would not guarantee natural movement in the fish.

Professor Sekine explaining operando spectroscopy
So, the idea is to go a step further and insert a small microsensor that will not affect the fish’s movement into its body. By transmitting radio signals from the microsensor, we can study the fish’s respiration, enabling us to do so while keeping the fish alive without it being affected by the monitoring. This monitoring method is called operando.
Similarly with a catalytic reaction, monitoring a phenomenon in its natural state leads to a true discovery. Analyzing test material taken from a laboratory is analogous to dissecting a dead fish. Instead, we can monitor the exact moment of the reaction, having inserted gases into a catalytic reactor when they react, by spectroscopy. This method applied to catalytic reactions is what we call operando spectroscopy. Using this method enables us to obtain data of a live phenomenon at various monitored instants.
Proton Hopping Becomes Visible
While conducting a series of experiments and gathering data using operando spectroscopy, we were gradually able to understand the mechanism of electrocatalytic reactions, in which protons move by a process called hopping via water attached to the surface of the catalyst.
Proton hopping is the repeated phenomenon of protons (H+), positively charged hydrogen (H) atoms, bonding with water molecules and causing the original bonds between the oxygen atoms (O) and hydrogen atoms (H) to break, allowing the protons to pass to neighboring water molecules. The protons can be seen to migrate quickly across the catalyst surface as if they are in a bucket relay.
We realized that the energy needed for a catalytic reaction is kept low due to the rapid movement of protons by this proton hopping, thereby accelerating the reaction at a lower temperature. It also became clear that protons colliding with molecules attached to the catalyst surface generate an irreversible process.
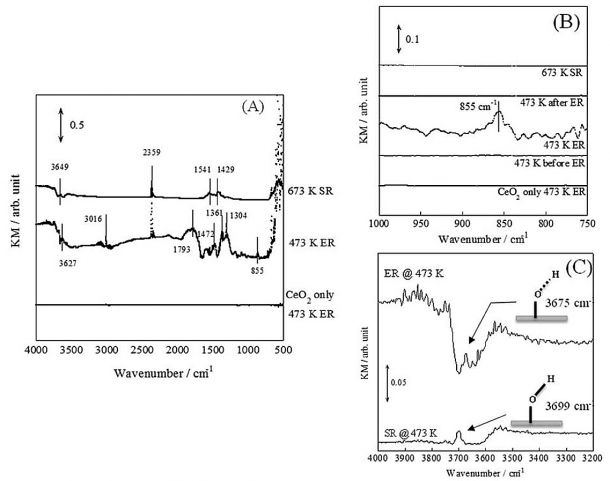
Figure: The operando-diffuse reflection method spectrum in the presence of an electric field. (A) is a comparison with and without palladium (Pd) catalyst and electric field application. KM represents the obtained spectrum converted to the Kubelka-Munk Formula. (B) shows the rotation range of oxygen (O) and hydrogen (H). (C) shows the expansion and contraction range of oxygen (O) and hydrogen (H). From these, we see that the reaction mechanism clearly varies depending on whether there is an electric field. (Source: The SEKINE group)

Figure: Diagram of a reaction proceeding by ions hopping on the surface. The surface is cerium monoxide (CeO), the large gray mass represents palladium (Pd), the red balls are hydrogen atoms or protons (H+), the pale yellow are oxygen (O) atoms, and the blue are carbon (C) atoms. The electric field is applied along the surface of the CeO. A water molecule (H2O) is hydrogen bonded to other neighboring water molecules. When it bonds to another water molecule with an H+ ion, its original O-H bond is broken and an H+ ion passes to the neighboring water molecule. If this is repeated, H+ ions can move extremely quickly across the CeO surface, rather like a bucket relay, even though the water molecules themselves do not move. H+ ions unilaterally collide with the Pd and react with the methane being held there, generating hydrogen molecules and carbon dioxide. (Source: The SEKINE Group)
These are completely new discoveries not published in any existing textbooks. Together with the students in my laboratory who were involved in this discovery, I published the article, “Surface Protonics Promotes Catalysis” in Scientific Report, the electronic journal of Nature magazine, in December 2016. The discovery of electrocatalytic reactions themselves, as introduced in Part 1, came about largely due to accidental factors. On the other hand, our discovery of the electrocatalytic reaction mechanism this time was due to the accumulation of data. While collecting data and plotting graphs, we conducted trial runs that incorporated various different changes and follow-up tests, and slowly but steadily, we reached our goal.
Spreading the Term “Electrocatalytic Reaction”
The method of producing catalytic reactions by applying an electric field has become popular across various fields, and the term “electrocatalytic reaction” is now used in many countries and academic papers. Even overseas, research related to electrocatalytic reactions is steadily underway. I am very pleased that researchers around the world have noticed how interesting this research is. However, the research requires advanced technologies, so studies on this topic is being conducted abroad only in countries such as the United States, Singapore, and some parts of Europe. At the moment, our laboratory is a step ahead because catalytic reactions and its mechanism are something that we discovered. Now that we know how it works, various applications of the mechanism are possible. I would like to talk about this more next time.
In Part 3, Professor Sekine shares his new research results with the key word being “hydrogen carrier,” something that stores and carries hydrogen, which is essential for the realization of a hydrogen society.
Profile
 Professor Yasushi Sekine
Professor Yasushi Sekine
Professor Yasushi Sekine obtained a Ph.D. (Doctor of Engineering) in 1998 in applied chemistry at the School of Engineering, the University of Tokyo. After serving as research associate in applied chemistry at the University of Tokyo, and research associate and assistant professor at Waseda University, he became professor at Waseda’s School of Advanced Science and Engineering in 2012. Since 2011, he has concurrently served as fellow of Japan Science and Technology Agency (JST). Professor Sekine has received awards such as the Japan Petroleum Institute Award for Distinguished Papers, the Advancement Prize of the Japan Institute of Energy, and the Research Advancement Prize of the FSRJ (Research Association for Feedstock Recycling of Plastics Japan). For further details, visit The SEKINE Group website.
Major Achievements
Publications
- Role of support lattice oxygen on steam reforming of toluene for hydrogen production over Ni/La0.7Sr0.3AlO3-d catalyst (Applied Catalysis A: General, 2013, 453, p60-70)
- Highly and stably dispersed Pt catalysts supported over La1-xSrxAlO3-0.5x perovskite for oxidative methane activation and their structures (Applied Catalysis A: General, 2013, 458, p71-81)
- Low-temperature catalytic oxidative coupling of methane in an electric field over a Ce-W-O catalyst system (Scientific Reports, 2016,6, 25154)
- Surface protonics promotes catalysis (Scientific Reports,2016, 6, 38007)
- Electro-catalytic synthesis of ammonia by surface proton hopping (Chemical Science, 2017, 8, p5434-5439)
Honors and Awards
- 2005 Japan Petroleum Institute Award for Encouragement of Research and Development
- 2007 Catalysis Society of Japan Award for Young Researchers
- 2009 Research Association for Feedstock Recycling of Plastics in Japan Most Progressive Researcher Award
- 2010 The Japan Institute of Energy Progressive Researcher Award (Academic)
- 2015 Japan Petroleum Institute Award for Distinguished Papers