Waseda Frontline Research Feature Vol. 19: Exploring Undiscovered Potential of Catalysts (Part 3 of 4)
Mon, May 14, 2018-
Tags
Yasushi Sekine
Professor, Department of Applied Chemistry, School of Advanced Science and Engineering, Faculty of Science and Engineering
Research Field: Catalytic Chemistry
Part 3: Building a Hydrogen Society with Hydrogen Carriers

Professor Yasushi Sekine was the first to establish a catalytic reaction for creating hydrogen by applying an electric field that exerts a slight electric force, and explained its mechanism. The results of his research can also be applied to methods of generating ammonia, a substance that stores and transports hydrogen. This time, he talks about his research and development of a “hydrogen carrier,” essential for the realization of a hydrogen society, focusing on ammonia and organic hydride as keywords. (Interviewed on September 7, 2017)
Ammonia, a Hydrogen Carrier
The full explanation of the phenomenon of electrocatalytic reactions introduced in Parts 1 and 2 has enabled other applications than just hydrogen generation. I was able to establish a method for efficiently producing ammonia, a leading candidate as a hydrogen carrier to store and transport hydrogen.
First, let me explain what hydrogen carriers are. Hydrogen molecules have a very low mass and exist as gas at room temperature. In its gaseous form, molecular hydrogen is very difficult to store and transport. For instance, you cannot just tie balloons containing hydrogen gas to a car and drive it around for transportation. So, perhaps bonding hydrogen to another element can convert it into a state that is easier to store and transport, such as liquid. Such a substance that stores and carries hydrogen is called a hydrogen carrier.
Using hydrogen carriers allows us to transport and even extract hydrogen when required. Previously, much research on storing hydrogen in metal took place, but because of technical challenges, such as the embrittlement of metals, very few metal storage options now remain. On the other hand, there is currently a lot of investigation into methods of storing hydrogen as liquid. As a liquid hydrogen carrier, ammonia is one of the best options.
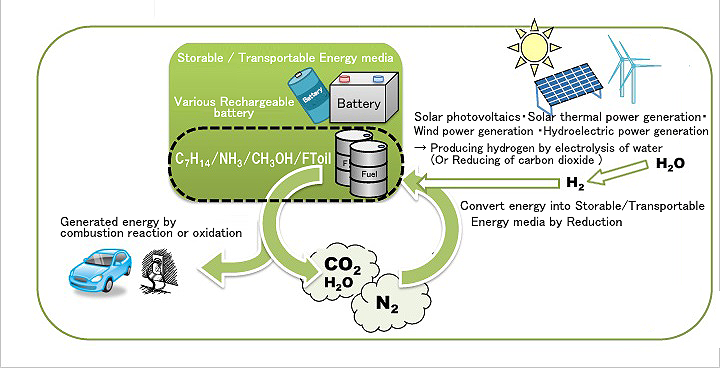
Figure: System combining natural energies with a hydrogen carrier (Source: The SEKINE Group)
Because ammonia (NH3) is composed of hydrogen (H) and nitrogen (N), the latter being abundant in the atmosphere, it is relatively easy to generate. Also, ammonia can be used as raw material, for instance, as fertilizer, and is a very beneficial substance for society to this day. The traditional method of generating ammonia was called the Haber-Bosch process, developed during World War I in the early 20th century. This process, which is still mainstream, uses a catalyst with iron as the main ingredient to produce ammonia from molecular nitrogen and molecular hydrogen. However, this method requires a temperature of 400 to 600°C to drive the catalytic reaction. It also requires the use of a high pressure environment. Considering how much generated heat is used, the method is efficient but requires a high temperature and high pressure environment. Because of this, technology that could combine low temperatures and high efficiency at smaller facilities to synthesize just the desired amount of ammonia was needed.
World’s Highest Ammonia Synthesis Rate
Having discovered electrocatalytic reactions, I took various opportunities to announce that catalytic reactions proceeded at lower temperatures when an electric field was applied. Around the same time, I was approached by a company working on new energies and catalysts who wondered whether electrocatalytic reactions could be utilized in the synthesis of ammonia. So, we began to carry out joint research.
At our laboratory, we searched for a catalyst that could be used in the synthesis of ammonia as an application of electrocatalytic reactions. We discovered that we could synthesize ammonium quickly and even at low temperatures of around 200°C by applying a weak electric field of a few Watts to a catalyst with atoms of the metal ruthenium (Ru) attached.
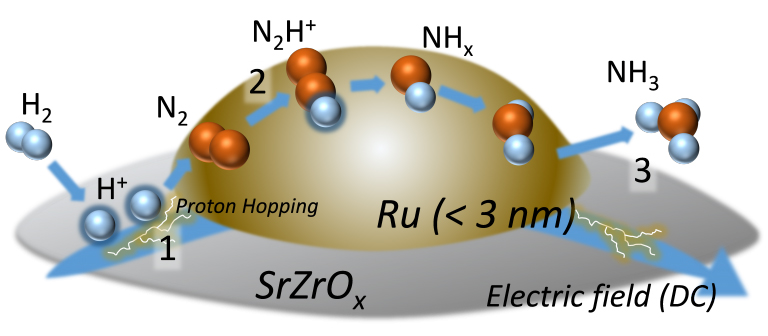
Figure: Diagram of the process of ammonia synthesis due to proton hopping produced by an electrocatalytic reaction Ru is a metal cluster of ruthenium. SrZrOx is a carrier with semiconductor properties. By applying an electric field, hydrogen ions hop across the surface (1 in diagram) and react with nitrogen molecules (N2) to form the intermediate N2H+ (2 in diagram) before forming ammonia (NH3) (3 in diagram).
Meanwhile, the company’s researchers had prepared internal experimental equipment with which to use our research results, and tried to make this catalytic system proceed under 9 atmospheres of pressure. In doing so, they achieved a world-leading rate of ammonia synthesis of 30 μmol/g in one hour.
We published these research results together with the company’s researchers and Professor Hiromi Nakai and his team at Waseda University in the British scientific journal Chemical Science under the title “Electrocatalytic synthesis of ammonia by surface proton hopping.” In future, by combining this technology with hydrogen generation technology that uses renewable energy, we expect to realize highly efficient ammonia synthesis plants with an output of dozens of tons or up to 100 tons per day.
Another Hydrogen Carrier, Organic Hydride
In addition to ammonia, there is another liquid substance that is highly expected to become a possible hydrogen carrier. It is a compound called organic hydride. In our laboratory, we are also studying organic hydride.
Hydrides are compounds made from hydrogen and another chemical element. A hydride is organic when hydrogen bonds with carbon (C), an organic substance. In other words, an organic hydride is a compound consisting of elemental carbon and elemental hydrogen. There are a number of different kinds of organic hydride, but the one that is being researched for use as a hydrogen carrier is a compound called methylcyclohexane (C6H11CH3).
The materials for making an organic hydride are hydrogen and a substance like petroleum. Completed, it can be thought of as a liquid like gasoline but containing hydrogen. For instance, when hydrogen is extracted from the organic hydride methylcyclohexane, the liquid becomes a substance called toluene, which, unlike when used as a fossil fuel, can be reused any number of times after hydrogen extraction. Also, this organic hydride can be kept in the home, unlike ammonia, which has a strong odor.
Society 100 Years from Now in Perspective
In R&D plans, for instance, in the cross-ministerial Strategic Innovation promotion Program (SIP) led by the Cabinet Office, ammonia is the strongest candidate as a hydrogen carrier while organic hydrides are positioned as the second. This focus is on which substances, including liquefied hydrogen and others, will be the main hydrogen carriers. However, although these hydrogen carriers are mutual rivals, they simultaneously complement one another too. That is to say, they can be used for different purposes depending on the application and the field.
When ammonia is used up, the only thing remaining is nitrogen. Nitrogen is a gas that is abundant in the atmosphere, so it can be released as it is. That is why ammonia is good for applications where it is used up on the spot. For example, on a remote island where wind power generation is the energy source, hydrogen could be made from water using an electrocatalytic reaction and then combined with nitrogen from the air to make ammonia. This means we could achieve a situation where a hydrogen carrier is made from existing local materials, and nothing is left over after the hydrogen is used.
As for organic hydrides, a liquid is left over after the hydrogen has been extracted, but this is suitable for transportation between destinations. For example, think about the means of transportation back and forth between Tokyo and Osaka. With organic hydride as a hydrogen carrier, the method is to carry it from the carrier factory in a coastal area to the point of use where hydrogen is extracted and used, then to carry the toluene produced back to the factory and combine it with fresh hydrogen, turning it back into organic hydride for reuse. Researchers and companies tend to take one of two positions, supporting either ammonia or organic hydride, but I think both are viable depending on the application. That is why I am studying both ammonia and organic hydride.
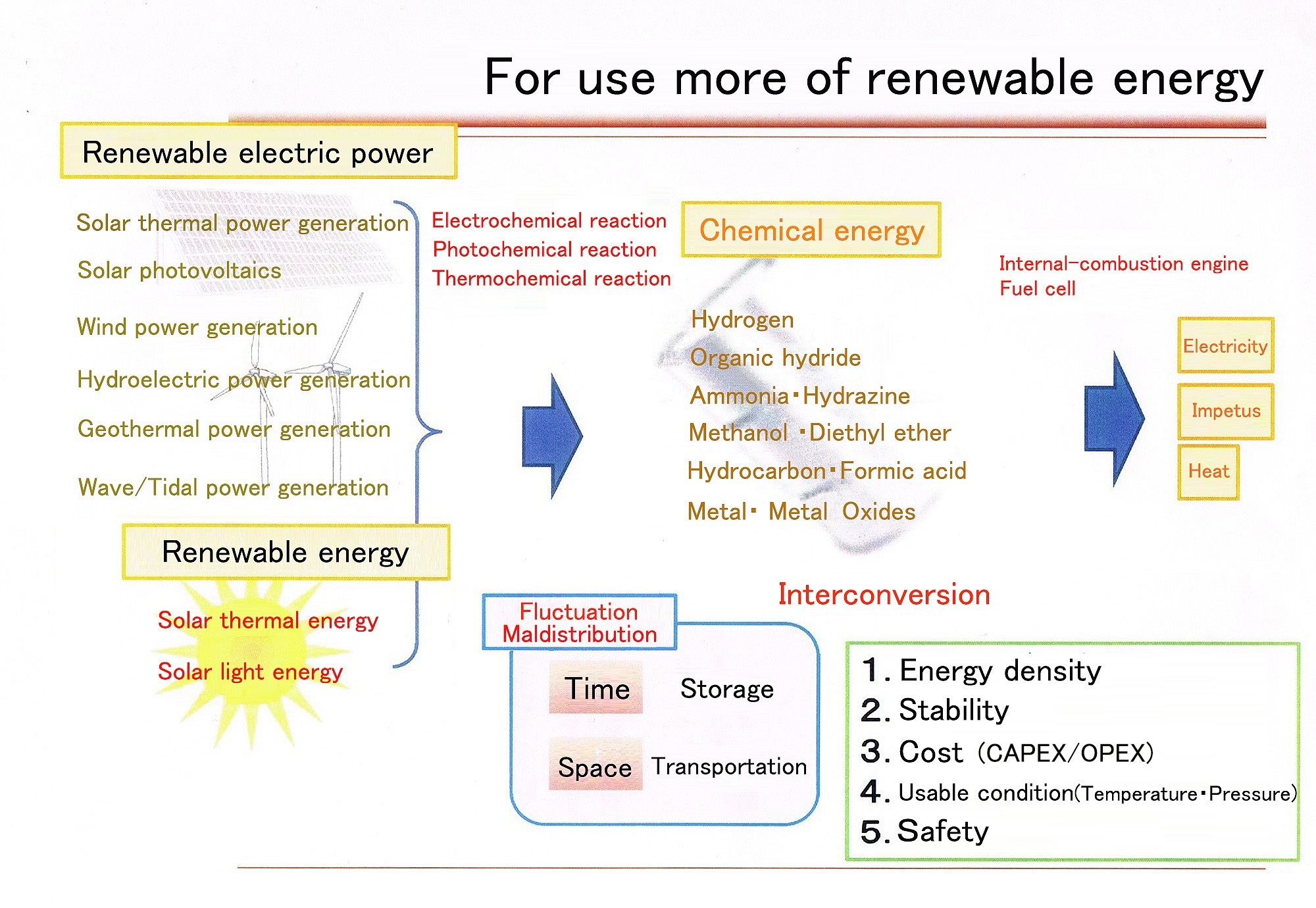
Figure: Vision for large-scale introduction of renewable energy (Source: The SEKINE Group)

In the laboratory, we conduct research with a long-term perspective that will lead to the construction of our future society.
I dare say that in about 100 years, our society will still be using fossil fuels. When those fossil fuels become depleted and the age of fossil fuels finally comes to an end, I hope the research I am working on will be of some help to society. I am pursuing my research with an eye on the future 100 years from now.
In the last part of the series, Professor Sekine will share his outlook on life, which has significantly influenced his approach to research. He will also talk about the advantage of conducting research at Waseda University.
Profile
 Professor Yasushi Sekine
Professor Yasushi Sekine
Professor Yasushi Sekine obtained a Ph.D. (Doctor of Engineering) in 1998 in applied chemistry at the School of Engineering, the University of Tokyo. After serving as research associate in applied chemistry at the University of Tokyo, and research associate and assistant professor at Waseda University, he became professor at Waseda’s School of Advanced Science and Engineering in 2012. Since 2011, he has concurrently served as fellow of Japan Science and Technology Agency (JST). Professor Sekine has received awards such as the Japan Petroleum Institute Award for Distinguished Papers, the Advancement Prize of the Japan Institute of Energy, and the Research Advancement Prize of the FSRJ (Research Association for Feedstock Recycling of Plastics Japan). For further details, visit The SEKINE Group website.
Major Achievements
Publications
- Role of support lattice oxygen on steam reforming of toluene for hydrogen production over Ni/La0.7Sr0.3AlO3-d catalyst (Applied Catalysis A: General, 2013, 453, p60-70)
- Highly and stably dispersed Pt catalysts supported over La1-xSrxAlO3-0.5x perovskite for oxidative methane activation and their structures (Applied Catalysis A: General, 2013, 458, p71-81)
- Low-temperature catalytic oxidative coupling of methane in an electric field over a Ce-W-O catalyst system (Scientific Reports, 2016,6, 25154)
- Surface protonics promotes catalysis (Scientific Reports,2016, 6, 38007)
- Electro-catalytic synthesis of ammonia by surface proton hopping (Chemical Science, 2017, 8, p5434-5439)
Honors and Awards
- 2005 Japan Petroleum Institute Award for Encouragement of Research and Development
- 2007 Catalysis Society of Japan Award for Young Researchers
- 2009 Research Association for Feedstock Recycling of Plastics in Japan Most Progressive Researcher Award
- 2010 The Japan Institute of Energy Progressive Researcher Award (Academic)
- 2015 Japan Petroleum Institute Award for Distinguished Papers













