Waseda Frontline Research Feature Vol.19: Exploring Undiscovered Potential of Catalysts (Part 1 of 4)
Mon, Mar 26, 2018-
Tags
Yasushi Sekine
Professor, Department of Applied Chemistry, School of Advanced Science and Engineering, Faculty of Science and Engineering
Research Field: Catalytic Chemistry
Part 1: Center of Chemistry

A catalyst is a substance that acts as an agent by speeding up a chemical reaction between other substances without changing itself. Many products that we use in our lives are made by reactions employing some kind of catalyst. Professor Yasushi Sekine is investigating undiscovered ways of using these catalysts for a better future. In this series, Professor Sekine talks about what catalytic chemistry is, the uniqueness of his research, and his outlook on life which influenced his approach to research. Part 1 focuses on the importance of catalysts and the details of his study on establishing an efficient method for generating hydrogen, using a catalyst. (Interviewed on September 7, 2017)
Catalyst Research is the Center of Chemistry
I specialize in catalytic chemistry.
Within the natural sciences, chemistry, alongside physics, is known as “the center of science.” In all areas of science and technology, including machinery, electricity, nuclear energy and architecture, a thorough investigation of the fundamental principles always leads us back to atomic- or molecular-level physics, such as chemical structure and intermolecular forces. Chemistry forms the basis of nearly all scientific and technological fields, which is why it is called the center of science.
And within chemistry, catalytic chemistry is considered as its center because chemical reactions that progress due to the effect of catalysts, that is, catalytic reactions, are extremely important processes in the chemical industrial field which sustains our daily lives. In fact, 90% of the chemical reactions used in the chemical industry are said to be catalytic reactions.
Japan buys a massive amount of energy of approximately 24 exajoules from abroad per year, 10 of which is procured as petroleum. Nearly all processes for manufacturing products from petroleum involve a catalyst. For example, when obtaining high-octane gasoline by distilling petroleum, a desulfurization catalyst is used to remove the sulfur content. Also, when gasoline is combusted to run automobiles, exhaust gas is treated with a NOx reduction catalyst to prevent the emission of nitrogen oxide, a cause of acid rain. Furthermore, plastics are made from petroleum in petrochemistry, and likewise, naphtha is made from distilling petroleum to lighten its relative density, which is then purified and modified by various catalysts.

Most large-scale chemical plants are operated using catalytic reactions
Catalysts are also used to produce familiar food products. For instance, perhaps many people prefer margarine on their bread. Margarine is made by hardening natural oils by catalyst-assisted hydrogenation. These are just a very few examples of the application of catalytic reactions. A great deal of our everyday life is dependent on the chemical industry, nearly all of which involves catalytic reactions. Catalysts may seem inconspicuous to most people, but it is no exaggeration to say that they prop up the very foundation of our society.
Hydrogen as an Energy Source
One of the things that can be generated using a catalytic reaction is hydrogen. It is said that the world of the future will be a “hydrogen society.” Until now, humankind has excavated fossil fuels that took about four billion years to make, such as oil, coal and natural gas, and used them in an instant. But there is no guarantee that fossil fuels can continue to be used. We have to think of ways to continue using energy long into the future.
When you consider which elements could be used as energy while looking at the rows of elements in the periodic table, you realize they are limited to carbon, lithium, hydrogen and a few others. Lithium is already used in batteries by utilizing oxidation-reduction reactions. When carbon is burned, it produces carbon dioxide. So, the only remaining element that can be burned to produce energy is hydrogen. As the fossil fuel draws to an end, the arrival of a hydrogen society is inevitable.
However, there are hardly any hydrogen molecules (H2) in the natural world. That is because hydrogen bonds easily with carbon to form water molecules, which are more stable. For this reason, we need to produce hydrogen by, for instance, splitting water molecules (H2O). This is done using a catalytic reaction. Until now, obtaining hydrogen molecules from water molecules has been done by reacting methane with steam using a catalyst.
CH4 + 2H2O → CO2 + 4H2
However, this method requires high temperatures of 700°C or more to speed up the catalytic reaction. The problem is that obtaining hydrogen as an energy source requires the use of a large amount of energy to raise the temperature. Preparing the materials is also difficult, so there is a lack of convenience in producing hydrogen whenever it is wanted. Because I had in mind to conduct unprecedented research, given this situation, I took on the challenge of establishing a method for obtaining hydrogen molecules from water molecules at temperatures between 150°C and 200°C, far lower than in conventional methods.

Professor Sekine
Electrocatalytic Reactions
In the early 2000s, I was engaged in unique research on the catalytic reaction produced where there is plasma. One day, while conducting an experiment with my students, I observed a reaction that made me wonder what had just happened. It seemed strange to me that even when the voltage was increased, the catalytic reaction did not follow and accelerate linearly. My students said that perhaps the reaction had not occurred simply because not much plasma had been generated, but I was doubtful. So, I instructed the students to try again so that there was no plasma. Surprisingly, when they did so, the catalytic reaction proceeded at much faster pace. In other words, we had found that weakening the applied voltage significantly strengthened the catalytic reaction. We obtained this result coincidentally while watching an experiment being conducted by my students.

In Professor Sekine’s laboratory at the Nishi-Waseda Campus. There is an array of devices producing various chemical reactions including catalytic reactions.
We had discovered that using a weak voltage rather than plasma or electrolysis to stage a catalytic reaction can produce hydrogen, a method which nobody had found before. Even at the low temperature of 150°C, the catalytic reaction proceeded substantially and hydrogen was generated. I named this method “electrocatalytic reaction” and presented it in 2009. The catalytic reaction proceeded at a low temperature with just a slight voltage, realizing high energy efficiency.
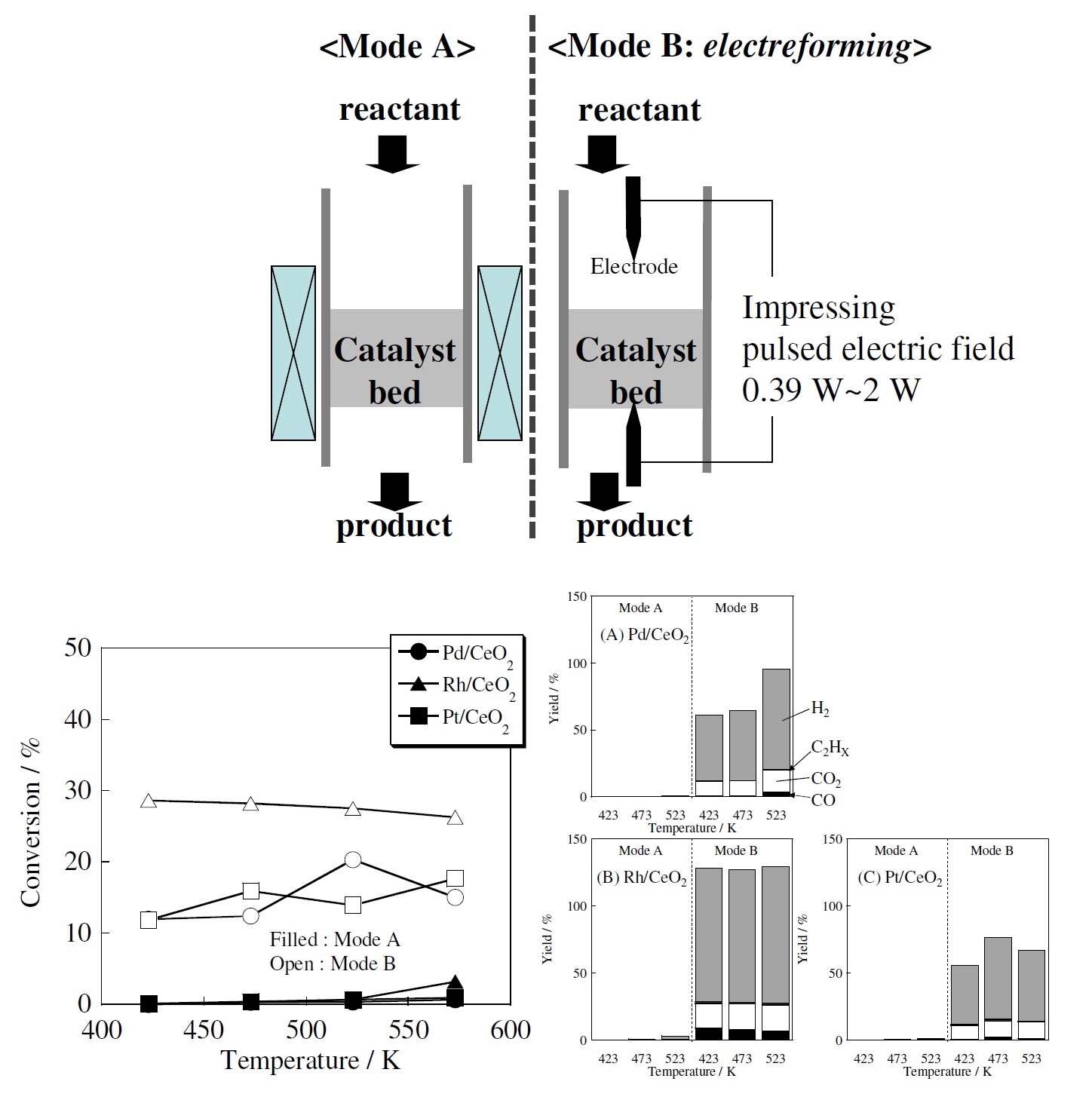
(Bottom left) Effect of an electric field in methane steam reforming (Bottom right) Difference in product using three kinds of catalyst at various temperatures by a conventional catalytic reaction and an electrocatalytic reaction (Source: The SEKINE Group)
Using an electrocatalytic reaction enables simple and convenient production of hydrogen when required. We have actually carried out joint research in this technology with more than ten companies. Some companies have even reached a practical application. I realized that we can produce a catalytic reaction much more efficiently than by generating plasma, so ever since this discovery, I have made electrocatalytic reactions the central theme of my research. One thing about electrocatalytic reactions remains a big mystery. That is the mechanism by which catalytic reactions are produced under weak voltage conditions. So next, I decided to try and explain this mechanism.
In Part 2, Professor Sekine talks about the path he took in order to unravel the mechanism of electrocatalytic reactions.
Profile
 Professor Yasushi Sekine
Professor Yasushi Sekine
Professor Yasushi Sekine obtained a Ph.D. (Doctor of Engineering) in 1998 in applied chemistry at the School of Engineering, the University of Tokyo. After serving as research associate in applied chemistry at the University of Tokyo, and research associate and assistant professor at Waseda University, he became professor at Waseda’s School of Advanced Science and Engineering in 2012. Since 2011, he has concurrently served as fellow of Japan Science and Technology Agency (JST). Professor Sekine has received awards such as the Japan Petroleum Institute Award for Distinguished Papers, the Advancement Prize of the Japan Institute of Energy, and the Research Advancement Prize of the FSRJ (Research Association for Feedstock Recycling of Plastics Japan). For further details, visit The SEKINE Group website.
Major Achievements
Publications
- Role of support lattice oxygen on steam reforming of toluene for hydrogen production over Ni/La0.7Sr0.3AlO3-d catalyst (Applied Catalysis A: General, 2013, 453, p60-70)
- Highly and stably dispersed Pt catalysts supported over La1-xSrxAlO3-0.5x perovskite for oxidative methane activation and their structures (Applied Catalysis A: General, 2013, 458, p71-81)
- Low-temperature catalytic oxidative coupling of methane in an electric field over a Ce-W-O catalyst system (Scientific Reports, 2016,6, 25154)
- Surface protonics promotes catalysis (Scientific Reports,2016, 6, 38007)
- Electro-catalytic synthesis of ammonia by surface proton hopping (Chemical Science, 2017, 8, p5434-5439)
Honors and Awards
- 2005 Japan Petroleum Institute Award for Encouragement of Research and Development
- 2007 Catalysis Society of Japan Award for Young Researchers
- 2009 Research Association for Feedstock Recycling of Plastics in Japan Most Progressive Researcher Award
- 2010 The Japan Institute of Energy Progressive Researcher Award (Academic)
- 2015 Japan Petroleum Institute Award for Distinguished Papers














