One-Step Route to Complex Molecules Using ortho-Quinodimethanes
Mon, Jun 16, 2025-
Tags
One-Step Route to Complex Molecules Using ortho-Quinodimethanes
Researchers develop a one-step method to generate ortho-quinodimethanes, solving a long-standing challenge in polycyclic synthesis
The Diels–Alder reaction is a widely used tool for building complex ring structures but often requires difficult-to-make starting materials. Researchers from Waseda University, along with other collaborators, developed a palladium-catalyzed method to generate reactive ortho-quinodimethanes using simple, commercially available compounds. This one-step, multicomponent reaction efficiently produces diverse polycyclic molecules. Their discovery of a unique benzyl–palladium intermediate offers a practical, faster approach to synthesizing biologically important and structurally complex compounds, including natural products.
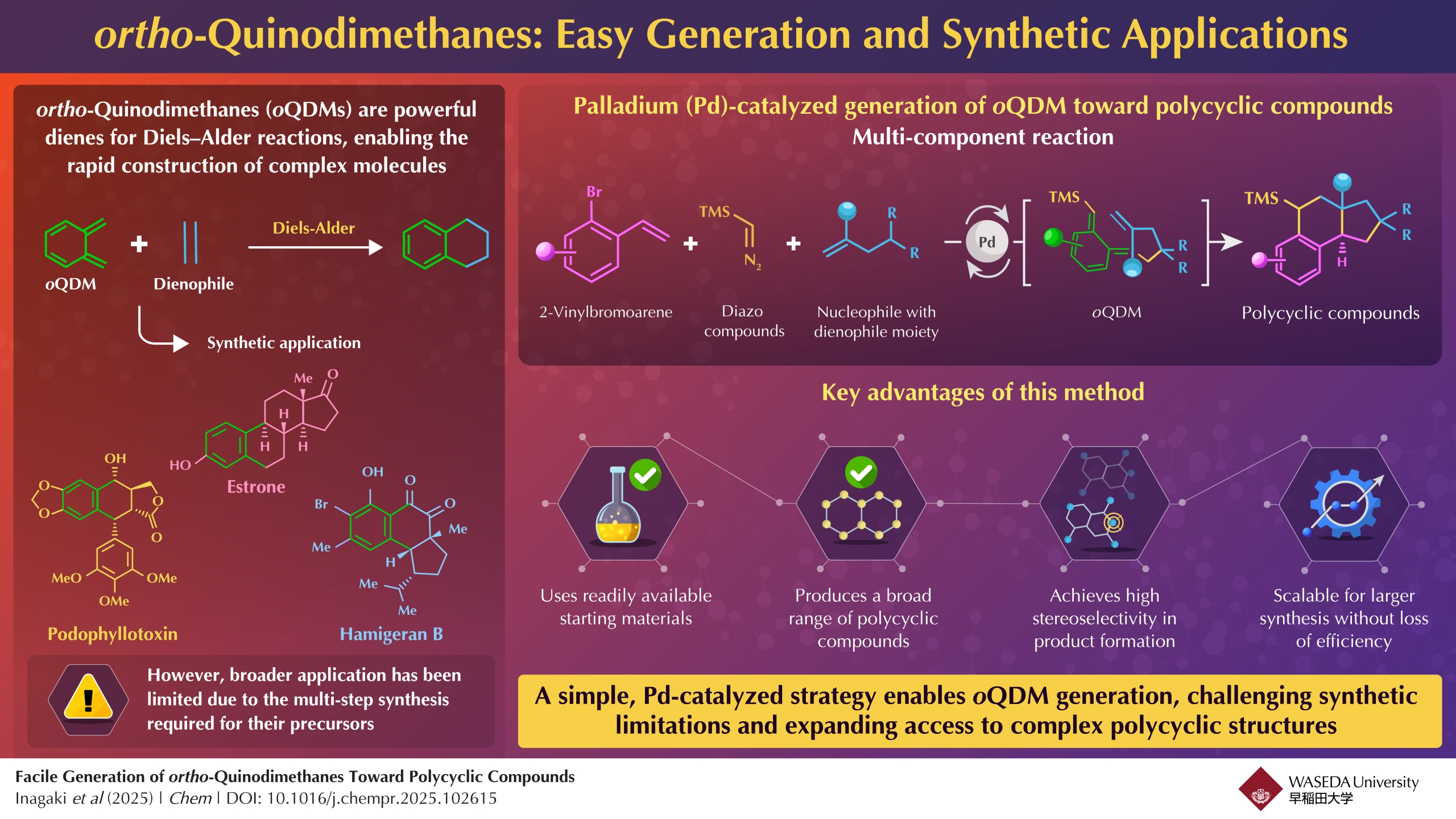
Image title: ortho-Quinodimethanes: Generation and Applications
Image caption: Researchers propose a facile method to generate ortho-quinodimethanes to eventually synthesize polycyclic compounds with biological applications.
Image credit: Professor Junichiro Yamaguchi from Waseda University, Japan
License type: Original content
Usage restriction: Cannot be reused without permission.
Organic chemistry features a wide array of reactions for creating complex molecules, among which the Diels–Alder reaction stands out for its versatility and precision. This reaction enables the construction of intricate polycyclic compounds—structures often found in natural products and pharmaceuticals—by joining dienes and dienophiles with high regio- and stereoselectivity.
One particularly valuable diene for this purpose is ortho-quinodimethane (oQDM), known for its ability to form fused-ring systems. However, synthesizing this reactive intermediate has traditionally required harsh conditions and the elaborate precursor preparation, limiting its practical use. This challenge has persisted for over 70 years in the field of organic synthesis.
Addressing this longstanding issue, a research team led by Professor Junichiro Yamaguchi at Waseda University, in collaboration with Dr. Kei Muto at the Institute of Transformative Bio-Molecules, Nagoya University, Japan, has developed a novel palladium (Pd)-catalyzed, multicomponent reaction. Their method uses readily available chemicals—2-vinylbromoarenes, diazo species, and carbon nucleophiles, containing a dienophile group—to generate oQDM and ultimately form a range of polycyclic compounds efficiently. Their findings were published in the journal Chem on June 2, 2025.
“The molecule oQDM has fascinated chemists for decades because of its potential to build complex structures, yet its instability has made it elusive,” says Yamaguchi. “We were motivated by the idea of transforming this fleeting intermediate into a practical synthetic tool. Inspired by how nature constructs complex molecules from simple components, we sought to replicate that elegance in the lab using catalytic control and accessible materials.”
In this study, the researchers developed a method that enables carbon–carbon bond formation via a highly reactive benzyl–Pd intermediate. This reactivity allows for the efficient construction of polycyclic structures with a vinyl group. The team demonstrated the broad applicability of the reaction by synthesizing a variety of complex compounds, including equilenin, a naturally occurring hormone-related molecule.
Their method significantly reduces the number of steps and harsh conditions typically required to access such compounds. As a result, this approach could make complex molecular structures more accessible for drug discovery and other applications. The researchers suggest that their reaction platform could be useful for building chemical libraries for drug screening and for developing new materials with functional properties.
“Our method enables access to molecular skeletons found in bioactive compounds, including hormone-based drugs and lead structures for anticancer and antiviral agents,” explains Yamaguchi. “It also allows for the rapid construction of compound libraries, which can support both pharmaceutical and materials research.”
This strategy offers a practical solution for synthesizing challenging polycyclic compounds while expanding the tools available to synthetic chemists working on applications in health and materials science.
Reference
Title of original paper:Facile Generation of ortho-Quinodimethanes Toward Polycyclic Compounds
DOI:10.1016/j.chempr.2025.102615
Journal: Chem
Article Publication Date: 2 June 2025
Authors: Kazuya Inagaki1, Yuna Onozawa1, Yuki Fukuhara1, Daisuke Yokogawa2, Kei Muto3, and Junichiro Yamaguchi1
Affiliation:
1Department of Applied Chemistry, Waseda University
2Graduate School of Arts and Sciences, The University of Tokyo
3Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University
About Professor Junichiro Yamaguchi from Waseda University
Professor Junichiro Yamaguchi is an organic chemist at the Faculty of Science and Engineering, Waseda University. His research focuses on creating efficient methods to construct complex molecules, especially polycyclic and aromatic compounds. He earned his Ph.D. from the Tokyo University of Science in 2007 and completed postdoctoral research at The Scripps Research Institute. He began his academic career at Nagoya University before joining Waseda in 2016 and becoming a professor in 2018. In addition to his research, Professor Yamaguchi is committed to science communication and leads Chem-Station, a popular chemistry portal that shares advanced science in engaging ways.