Revolutionizing Cancer Treatment by Intracellular Protein Delivery Using Hybrid Nanotubes
Mon, May 20, 2024-
Tags
Revolutionizing Cancer Treatment by Intracellular Protein Delivery Using Hybrid Nanotubes
The hybrid nanotube stamp system revolutionizes precision medicine with high efficiency and cell viability rates for cancer treatment
The intracellular delivery of proteins is an important technique for unveiling the cellular functions, protein complex structure, and therapeutics. However, the conventional delivery methods have several limitations. To address this, researchers from Japan have developed a novel hybrid nanotube (HyNT) stamp system that can deliver multiple proteins with high efficiency and viability rates. This system represents a groundbreaking advancement in intracellular protein delivery, offering precise injection of therapeutic agents into target cells.
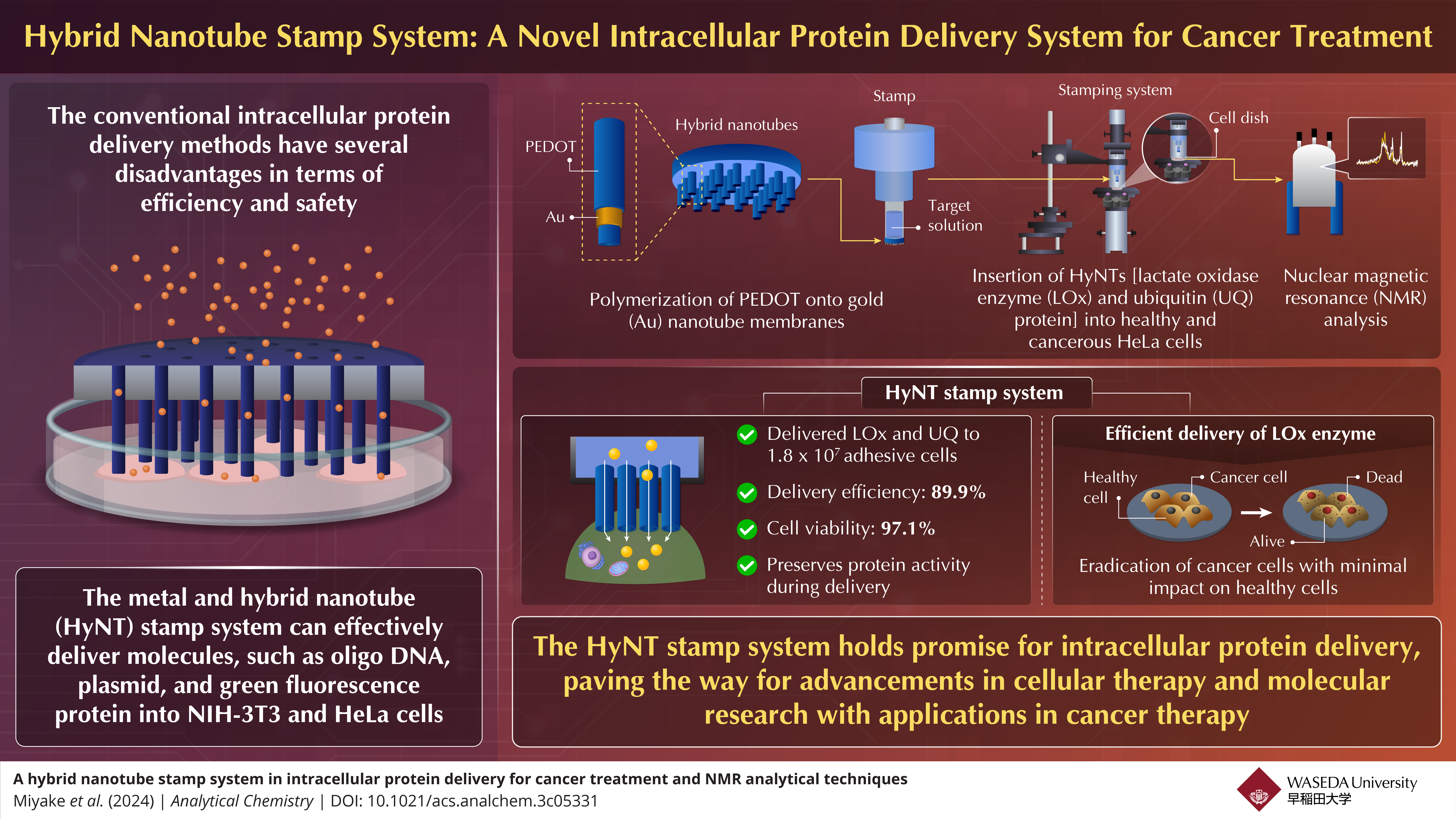
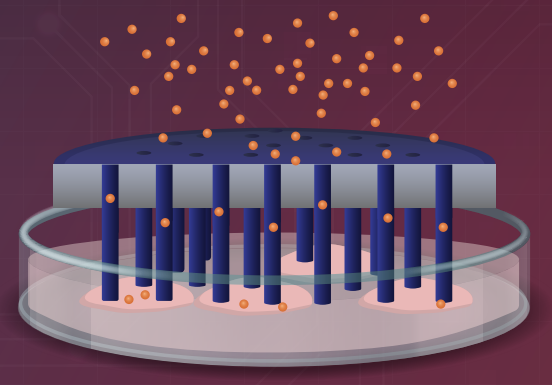
Image title: PEDOT/Au HyNT stamp system for intracellular protein delivery
Image caption: The HyNT stamp system demonstrates efficient delivery of molecules into cells, offering promising prospects for cancer therapy while ensuring high cell viability.
Image credit: Takeo Miyake from Waseda University
License type: Original Content
Usage restrictions: Cannot be reused without permission
In today’s medical landscape, precision medicine and targeted therapies are gaining traction for their ability to tailor treatments to individual patients while minimizing adverse effects. Conventional methods, such as gene transfer techniques, show promise in delivering therapeutic genes directly to cells to address various diseases. However, these methods face significant drawbacks, hindering their efficacy and safety.
Intracellular protein delivery offers a promising approach for developing safer, more targeted, and effective therapies. By directly transferring proteins into target cells, this method circumvents issues such as silencing during transcription and translation and the risk of undesirable mutations from DNA insertion. Additionally, intracellular protein delivery allows for precise distribution of therapeutic proteins within target cells without causing toxicity.
A group of researchers led by Professor Takeo Miyake at Waseda University, Japan in collaboration with the Mikawa Group at the RIKEN Institute have now developed a hybrid nanotube stamp system for intracellular delivery of proteins. This innovative technique enables the simultaneous delivery of diverse cargoes, including calcein dye, lactate oxidase (LOx) enzyme, and ubiquitin (UQ) protein, directly into adhesive cells for cancer treatment. An article describing their research was published in Analytical Chemistry on May 14, 2024. This article has been co-authored by Dr. Tsutomu Mikawa, Dr. Masaomi Ikari, Dr. Hiromasa Yagi, Dr. Naoya Tochio, and Dr. Takanori Kigawa from RIKEN Center for Biosystems Dynamics Research, Japan and Mr. Bowen Zhang, Mr. Bingfu Liu, Mr. Zhouji Wu, and Mr. Kazuhiro Oyama from Waseda University, Japan.
Miyake briefly explains the stamp system assembly. “The HyNTs were synthesized through PEDOT polymerization onto Au nanotube membranes, and then assembled with a glass tube to create a stamp capable of physically inserting HyNTs into cells.”
The researchers explored the therapeutic potential of delivering LOx enzyme for cancer treatment. “Through our innovative stamp system, we successfully delivered LOx into both healthy mesenchymal stem cells (MSC) and cancerous HeLa cells. While MSC cells remained unaffected, we observed significant cell death in HeLa cancer cells following LOx treatment with viabilities decreasing over time. Our findings highlight the promising efficacy of intracellularly delivered LOx in selectively targeting and killing cancer cells, while sparing healthy cells, offering a targeted therapeutic strategy for cancer treatment” explains Miyake.
Finally, the team successfully delivered 15N isotope-labeled UQ proteins into HeLa cells using the HyNT stamp system. This delivery allowed for the analysis of complex protein structures and interactions within the cells. In addition, optical and fluorescence imaging confirmed the presence of delivered UQ in HeLa cells, and nuclear magnetic resonance spectroscopy matched the intracellular UQ protein concentration with that of a solution containing 15N-labeled UQ. These results demonstrate the effectiveness of the stamp system in delivering target proteins for subsequent analysis.
The results demonstrate the remarkable capability of the HyNT stamp system in delivering LOx and UQ into a substantial number of adhesive cells, as required for regenerative medicine applications. The system achieved a notably high delivery efficiency of 89.9%, indicating its effectiveness in transporting therapeutic proteins into the target cells with precision. Moreover, the cell viability rate of 97.1% highlights the system’s ability to maintain the health and integrity of the treated cells throughout the delivery process.
The HyNT stamp system offers transformative potential in intracellular protein delivery, with applications spanning from cancer treatment to molecular analysis. Beyond medicine, its versatility extends to agriculture and food industries, promising advancements in crop production and food product development. With precise cell manipulation and efficient delivery, the HyNT stamp system is poised to revolutionize biomedical research, clinical practice, and diverse industries, paving the way for personalized interventions and shaping the future of modern medicine.
Reference
Authors
Bowen Zhang1,2, Bingfu Liu1, Zhouji Wu1, Kazuhiro Oyama1,2, Masaomi Ikari2, Hiromasa Yagi2, Naoya Tochio2, Takanori Kigawa2, Tsutomu Mikawa2, and Takeo Miyake1
Affiliations
1Graduate School of Information, Production and Systems, Waseda University, Japan
2RIKEN Center for Biosystems Dynamics Research, Japan
Title of original paper
Journal
Analytical Chemistry
DOI
About Professor Takeo Miyake
Dr. Takeo Miyake currently serves as a Professor and Associate Dean at Waseda University. He received B.S., M.S., and Ph.D. degrees from Waseda University between the years 2004 and 2008. His research is centered around the development of safe and soft bioelectronics that form seamless interfaces between devices and humans, and he has been actively researching and developing smart contact lenses for eye health monitoring and protection. Dr. Miyake believes that, soon, smart contact lens devices will become a tangible and inseparable part of modern humans, and thus wishes to be a pioneer in this movement.
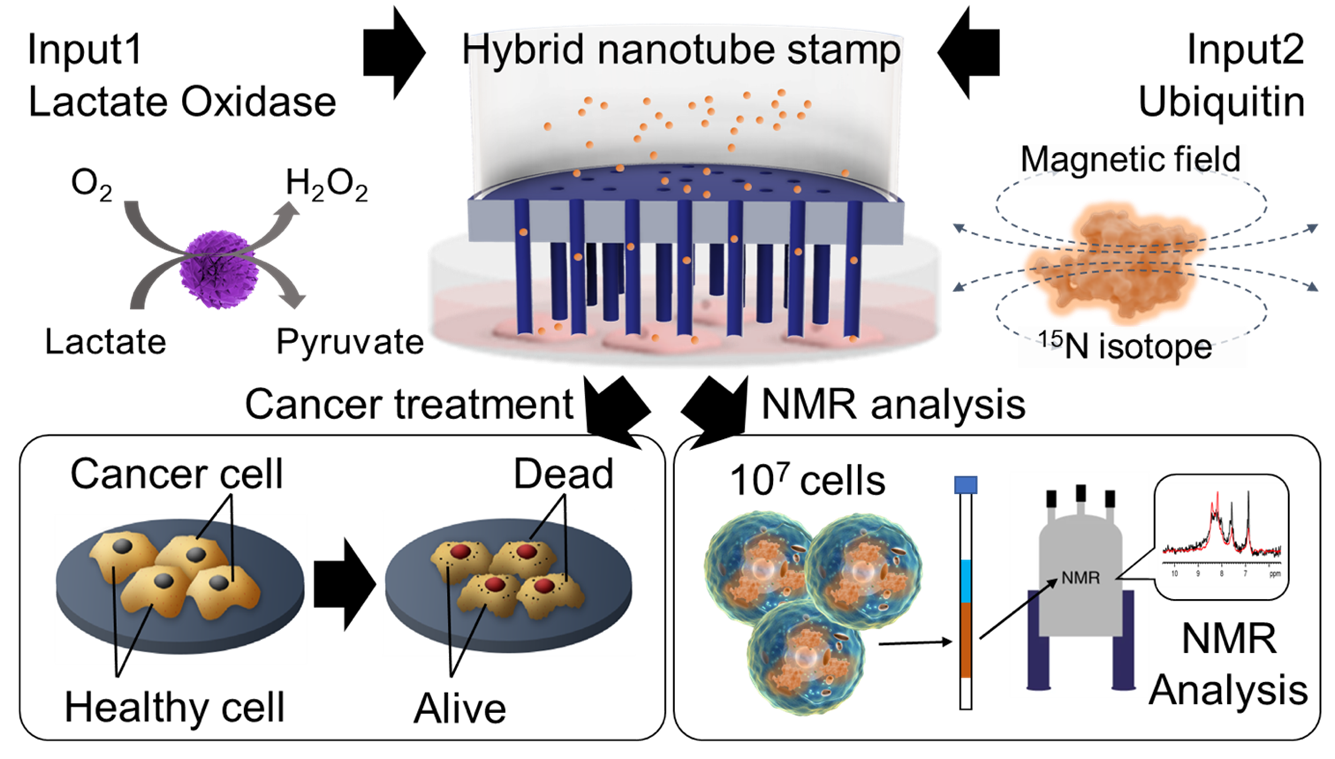
Image title: Hybrid nanotube stamp system for cancer treatment
Image caption: The HyNT stamp system effectively delivers LOx and UQ to 1.8 × 107 adhesive cells with a viability of 97.1%. This system through the delivery of LOx enzyme can effectively eradicate cancerous cells with minimal impact on healthy cells.
Image credit: Takeo Miyake from Waseda University
License type: Original Content
Usage restrictions: Cannot be reused without permission