Synthesizing 7-ring helicene in just two steps
Fri, Jul 14, 2017-
Tags
Great potential as CPL-emitting material for future communication devices and 3D displays
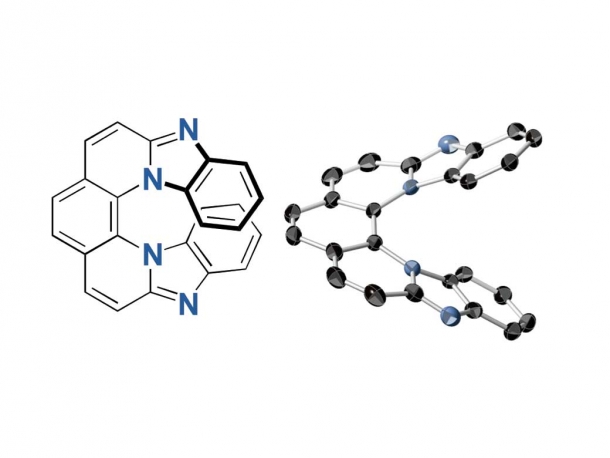
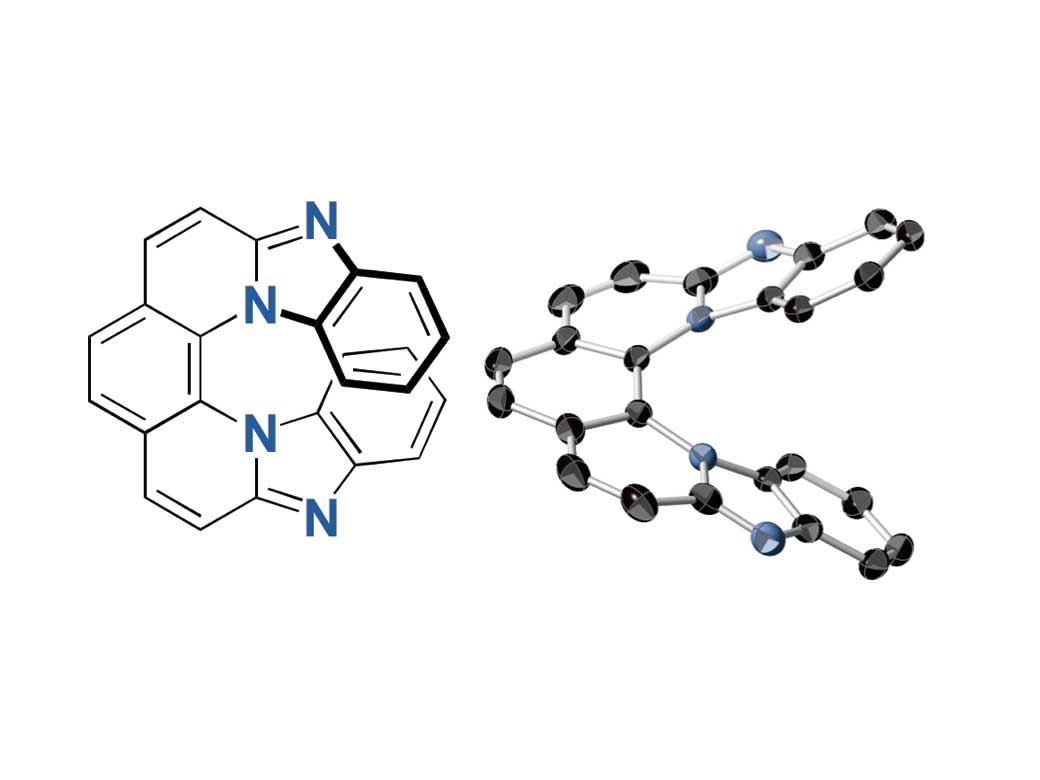
7-ring helicene
Helicene is a compound comprised of planar aromatic molecules such as benzene rings. When many benzene rings bond together, a 3D screw-shaped helical structure is formed to avoid clashing of the terminal rings known as steric hindrance. As a result, the structure endows chirality, a geometric property that is non-superposable on its mirror image.
Unlike other compounds, chiral helicenes exhibit unique optical properties, so in recent years, applications of helicenes in organic optoelectronics and electronics such as nonlinear optics and organic semiconductors have been investigated. Currently, 6-ring helicenes are considered the most stable, but there has been demand for helicenes with more rings for more chiral structures. In previous approaches, however, more than five steps were required for synthesizing helicenes, and its fluorescence, especially ones with seven rings or more, were generally low.
“In our study, we succeeded in a two-step synthesis of 7-ring helicenes with the highest fluorescence and CPL efficiency that have been reported to date, using a commercially available reagent,” says Takanori Shibata, professor of synthetic chemistry a Waseda University.
“Helicenes have potential applications in organic luminescent materials, such as a circularly polarized luminescence (CPL)-emitting material. Because CPL-emitting material adds on much more information, there are great expectations for developments in future communication devices and 3D displays.”
This research was published in Angewandte Chemie International Edition.
Researchers used 2,9-dichloro-1,10-phenanthroline for the synthesis and found that the 7-ring helicenes constitute a unique structure with a significantly twisted center and planar terminals through single-crystal X-ray analyses. They have also shown high fluorescence of 0.8 quantum yields and high CPL activity of glum up to 0.009, the highest glum value ever reported. Furthermore, the helicenes conserved its helical structure in high heat and contain nitrogen atoms, demonstrating stability against heat and under both neutral and acidic conditions.
The same method used to create the 7-ring helicenes could be applied for synthesis of polyzahelicenes of more than seven rings and provide new insight into the design of small CPL-emitting molecules, future material for 3D displays, security paint, and other optical information technology.
Reference
- Published in: Angewandte Chemie International Edition
- Title: Facile Two-Step Synthesis of 1,10-Phenanthroline-Derived Polyaza[7]helicenes with High Fluorescence and CPL Efficiency
- Authors: Takashi Otani, Ami Tsuyuki, Taiki Iwachi, Satoshi Someya, Kotaro Tateno, Hidetoshi Kawai, Takao Saito, Kyalo Stephen Kanyiva, and Takanori Shibata
- DOI: 10.1002/anie.201700507