Understanding Sex-Based Differences and the Role of Bone Morphogenetic Protein Signaling in Alzheimer’s Disease
Fri, Jan 9, 2026-
Tags
Understanding Sex-Based Differences and the Role of Bone Morphogenetic Protein Signaling in Alzheimer’s Disease
Researchers discover sex-related upregulation of bone morphogenetic protein signaling inhibits adult neurogenesis in Alzheimer’s disease
Alzheimer’s disease (AD) is a serious neurodegenerative disease largely affecting older adults. Apart from age, it also shows sex-based differences, with women being more at risk. However, the origin of these differences remains unknown. While bone morphogenetic proteins (BMPs) play an important role in adult neurogenesis, their role in AD remains elusive. To address this, researchers have investigated sex-based differences and role of BMP signaling in neurogenesis in AD mice models, uncovering novel therapeutic targets.
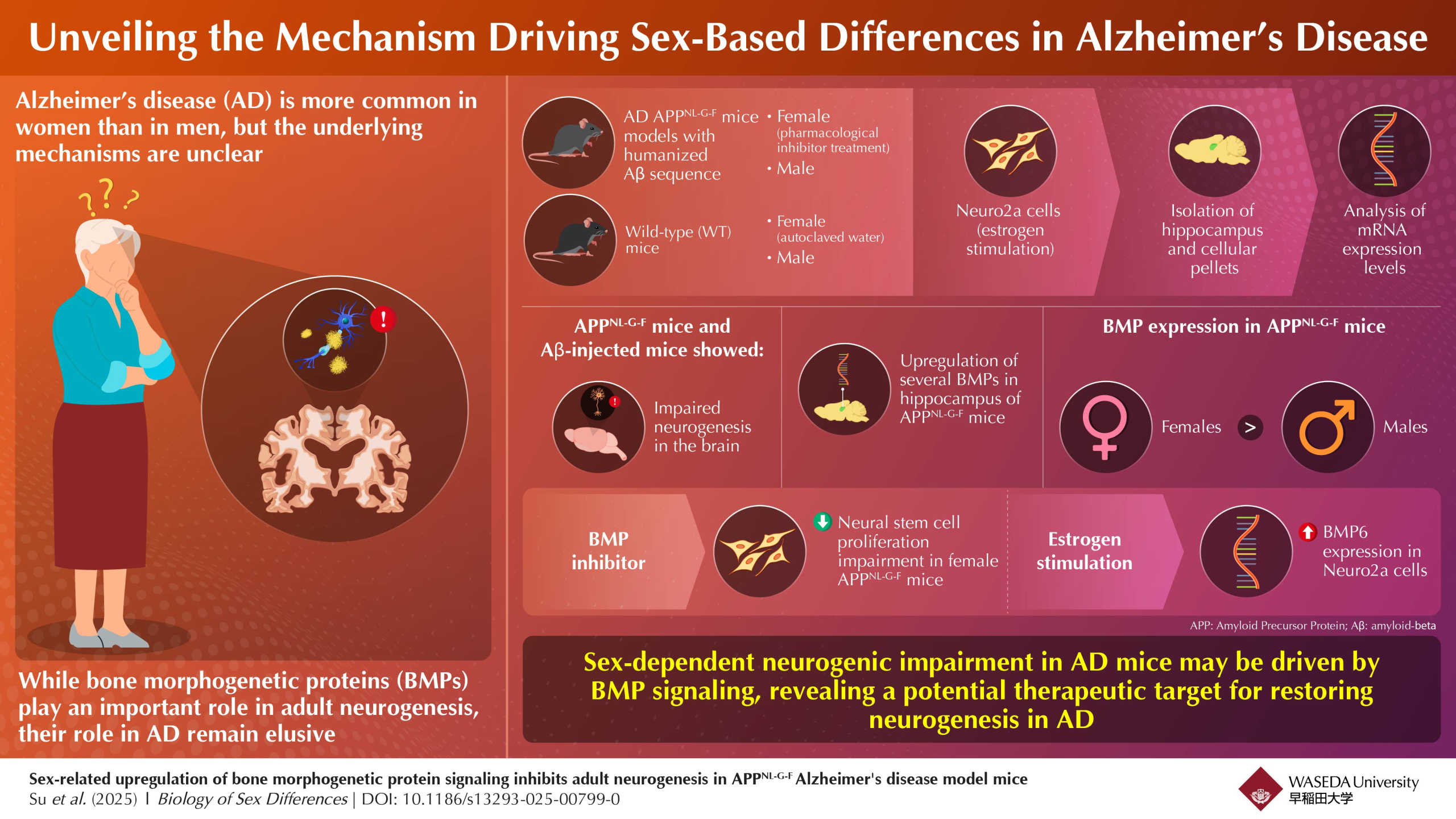
Image title: Origin of sex-based differences and role of bone morphogenetic protein (BMP) signaling in Alzheimer’s disease (AD)
Image caption:Researchers have discovered that sex-related upregulation of BMP signaling inhibits adult neurogenesis in AD.
Image credit: Xingyu Su from Waseda University, Japan
License type: Original content
Usage restrictions: Cannot be reused without permission.
Alzheimer’s disease (AD) is one of the main causes of dementia, characterized by progressive neurodegeneration, typically beginning at or after 65 years of age. The global incidence of AD is projected to increase in the coming decades, posing significant challenges to public health systems, families, caregivers, and socioeconomic structures. Currently, there is no cure for AD. While age is the greatest risk factor for AD, studies also show that more women than men suffer from AD, especially those over 85 years of age. However, the underlying biological mechanisms of these sex-based differences are poorly understood.
AD patients exhibit impaired neurogenesis. Recent evidence suggests that increased levels of bone morphogenetic proteins (BMPs), such as BMP6, in the brains of patients with AD and AD mice models, are linked to this impaired neurogenesis. BMPs are essential for patterning, development, and functioning of the central nervous system. Despite growing evidence of BMP involvement, how exactly BMP signaling influences AD remains unclear.
To address these gaps, a research team led by Xingyu Su, a PhD student from the Department of Life Science and Medical Bioscience, Faculty of Advanced Science and Engineering, Waseda University, Japan, investigated the role of BMP signaling in neurogenic impairment and sex-based differences in AD mice models. The team included Dr. Rina Takayanagi, Dr. Hiroki Maeda, and Dr. Toshio Ohshima from Waseda University, along with Dr. Takaomi C. Saido from the RIKEN Center for Brain Science, Japan. The study was published in Volume 16 of Biology of Sex Differences on December 12, 2025.
“AD disproportionately affects women, who also experience more severe cognitive decline than men. We wanted to investigate what drives these differences. From a scientific perspective, clarifying the exact role of BMP signaling is important for identifying new therapeutic targets. Thus, the motivation behind our study was both scientific and societal considerations,” says Su.
The researchers established two AD mice models: 6-month-old Amyloid Precursor Protein (APP)NL-G-F transgenic mice that carry the humanized amyloid-β (Aβ) sequence with three AD mutations, and age- and sex-matched C57BL/6J mice termed wild-type (WT). BMP expression in the hippocampus was assessed through quantitative real-time polymerase chain reaction, while neural stem cell proliferation was investigated through immunofluorescence staining.
Both the APPNL-G-F mice and Aβ-injected mice exhibited impaired neurogenesis in the brain. Additionally, in both mice models BMP4, BMP6, and BMP7, expression was significantly increased compared with WT controls. Notably, clear sex-based differences were found only in the APPNL-G-F mice, with female mice showing more severe neurogenesis impairment and higher BMP expression than males. These results indicate that activation of BMP signaling might be linked to impaired neurogenesis in AD.
To further investigate the relationship between BMP signaling and impaired neurogenesis, the researchers treated female APPNL-G-F mice with a pharmacological inhibitor of BMP. The treatment effectively alleviated neurogenic impairment in these mice and even restored neurogenesis to levels comparable to those in the WT control mice.
The researchers also conducted in vitro experiments with Neuro2a cells to understand the origin of observed sex-based differences. Neuro2a is a mouse neuroblast cell line that can differentiate into neuron-like cells and is known to express estrogen receptors. Interestingly, the team found that stimulating these cells with estrogen markedly increased BMP6 levels, providing a mechanistic link that may explain sex differences in AD.
“Our study provides new insights into the role of BMP signaling activation in impaired neurogenesis in AD, which will guide future translational research. Ultimately, this knowledge can lead to the development of novel intervention strategies, reducing the clinical and caregiving burdens associated with AD,” concludes Su.
Reference
|
Title of original paper: |
Sex-related upregulation of bone morphogenetic protein signaling inhibits adult neurogenesis in APPNL-G-F Alzheimer’s disease model mice |
|
Authors |
Xingyu Su1, Rina Takayanagi1, Hiroki Maeda1, Takaomi C. Saido2, and Toshio Ohshima1,3 |
|
Affiliations |
1Department of Life Science and Medical Bioscience, Waseda University |
| Latest Article Publication Date: |
12 December 2025 |
|
Journal: |
Biology of Sex Differences |
|
DOI: |
About Xingyu Su
Xingyu Su is currently a PhD student at the Department of Life Science and Medical Bioscience, Faculty of Advanced Science and Engineering, Waseda University, Japan. Her research focuses on understanding molecular mechanisms of neuronal differentiation, the brain’s wiring, and its functional development.
About Professor Toshio Ohshima
Toshio Ohshima, MD and PhD, is currently a Professor at the Department of Life Science and Medical Bioscience, Faculty of Advanced Science and Engineering, Waseda University, Japan. His research focuses on understanding molecular mechanisms of neuronal differentiation and regeneration.