Creating ethers from esters? New method for developing anti-cancer drugs and antimalarial medications
Mon, Feb 27, 2017-
Tags
Success in world’s first decarbonylative diaryl ether synthesis by Pd and Ni catalysis
 Researchers developed a catalytic decarbonlyative etherification of aromatic esters using a nickel or palladium catalyst with their enabling diphosphine ligand to give the corresponding diaryl ethers. In other words, they succeeded in developing the world’s first catalyst for removing carbon from esters, acid-derived chemical compounds where one or more OH group is replaced by an O-alkyl group, to create ethers, organic compounds with an oxygen atom attached to alkyl or aryl groups. This is the first of its kind in the world and provides a new method for pharmaceutical research in developing anti-cancer drugs and antimalarial medications.
Researchers developed a catalytic decarbonlyative etherification of aromatic esters using a nickel or palladium catalyst with their enabling diphosphine ligand to give the corresponding diaryl ethers. In other words, they succeeded in developing the world’s first catalyst for removing carbon from esters, acid-derived chemical compounds where one or more OH group is replaced by an O-alkyl group, to create ethers, organic compounds with an oxygen atom attached to alkyl or aryl groups. This is the first of its kind in the world and provides a new method for pharmaceutical research in developing anti-cancer drugs and antimalarial medications.
A paper on this research was published in the online version of Journal of the American Chemical Society on February 21.
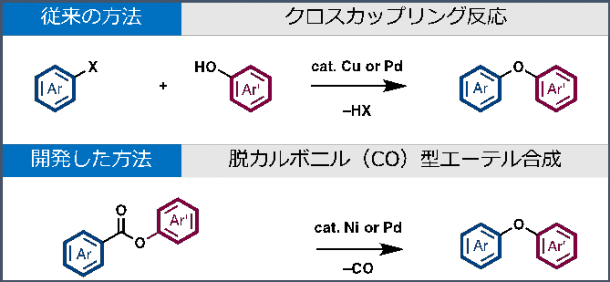
Above: The conventional method of intermolecular cross-coupling reaction of aryl halides and phenols with a copper or palladium catalyst Below: The newly developed method using nickel or palladium catalyst
Intermolecular cross-coupling reaction of aryl halides and phenols with a copper or palladium catalyst is the conventional method for constructing a diaryl ether functional group, but due to the costly expenses and concerns regarding waste management, there has been a demand for a more effective new method.

Decarbonylative ether synthesis by nickel or palladium catalysis
In this research, the catalyst successfully removed carbon monoxide from aromatic esters to form the Ar-O-Ar’ decarbonlyative diaryl ether synthesis. Using this method, achieving diaryl ethers from over 30 different kinds of aromatic esters becomes possible.
As a result, inexpensive and easily obtainable aromatic esters can synthesize diaryl ethers, contributing to future developments in pharmaceutical products.
For more information on this research, contact Junichiro Yamaguchi, associate professor in the Department of Applied Chemistry at Waseda University.
On the publication
- Journal: Journal of the American Chemical Society
- Title: Decarbonylative Diaryl Ether Synthesis by Pd and Ni Catalysis
- Authors: Ryosuke Takise, Ryota Isshiki, Kei Muto, Kenichiro Itami, and Junichiro Yamaguchi
- Published on: (Just Accepted Manuscripts)
- DOI: 10.1021/jacs.7b00049













