New decontamination tool – Catenaccio PA expected to help Fukushima cleanup
Wed, Jan 14, 2015-
Tags
An inexpensive and highly durable new material, it is hoped that Catenaccio PA will prove effective for treating contaminated water at the Fukushima Daiichi plant.
The Research Institute for Sustainable and Environmental Technology of Waseda University (RISET; Shinjuku Ward, Tokyo; Director: Prof. Masahiko Matsukata) and AZMEC Inc. (Headquarters: Mino City, Gifu Prefecture; President: Takenori Shoda) have successfully developed a new material for adsorbing radioactive elements such as cesium and strontium. An inexpensive and highly durable adsorbent, Catenaccio PA is a huge innovation over existing materials. With the Fukushima Daiichi Nuclear Power Station still facing the question of how to tackle the heavily contaminated discharge water at the plant, this highly versatile adsorbent, which is not only able to treat large volumes of contaminated water but which can also be used in other applications such as interim storage facilities for radioactive waste, is expected to have a far-reaching impact.
Traditional adsorbents for radioactive elements have been extremely expensive (costing tens of thousands of yen per kilogram); natural zeolite, by contrast, is low in cost but its adsorption performance is poor, and it also has been known to break down and lose effectiveness in highly alkaline environments such as ash. Catenaccio PA, however, is an inorganic polymer-based adsorbent made from silica and aluminum. As its raw materials are extremely low in cost (around 600 yen per kilogram) it is very easy to manufacture. It also has excellent acid resistance and alkali resistance. In addition, it can be used in interim storage facilities for long periods of time, lasting for at least 30 years before replacement is necessary.
RISET developed this material after its attention was drawn to the cation exchange capacity (CEC) of geopolymer, an inorganic substance which is used as a substitute for cement. Noting the material’s CEC level, the researchers decided to make use of this feature. Catenaccio PA is expected to make a significant contribution towards improved safety not only through its use in the treatment of contaminated water from nuclear power plants but also in preventing radiation leaks from interim waste storage facilities currently being planned.
Researchers leading development of Catenaccio
- Atsushi Yamazaki, Professor, Faculty of Science and Engineering
- Takenori Shoda, President, AZMEC Inc.
- Masahiko Matsukata, Professor, Faculty of Science and Engineering; Director, RISET
Inquiries: Mr. Matsuoka, Waseda University RISET (03-5292-1661)
(1) Summary of past research
It is a well known that most of the cesium and strontium adsorbents which have been used up until now are expensive, with strontium adsorbents in particular being extremely high in price. While natural zeolite is low in cost, it too has problems: its adsorption performance is low, and it can dissolve and lose effectiveness when exposed to high levels of alkalinity.
(2) Objectives of this research
The goal was to develop a low-cost high-performance adsorbent for both cesium and strontium, which could be used without problems in high-alkalinity environments (i.e. with excellent durability), and be viable as commercial products.
(3) New methods developed for this research
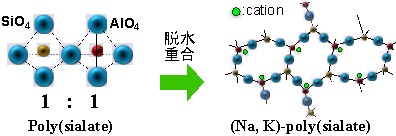
Geopolymer is an inorganic polymer consisting primarily of silica and aluminum. Research both inside and outside Japan had already looked at geopolymer as a substitute for cement, and the product had been partially commercialized. However, almost all geopolymer was being used as a cement substitute. CEC is a characteristic of geopolymer, yet up to this point relatively little consideration had been given to making use of this function. The adsorbent created through this research—an inorganic polymer-based adsorbent (see Fig. 1)—was developed through focusing on this characteristic.
(4) Outcomes and findings of this research
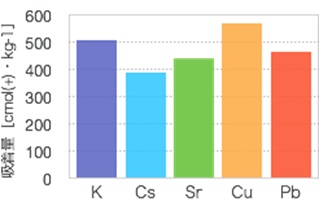
By testing the raw materials and adjusting the silica-to-aluminum ratio, an adsorbent was created which combines the ion-exchange capability and adsorptive immobilization of zeolite with the acid/alkali resistance inherent to polymers. This product has been commercialized and launched under the name Catenaccio PA. These adsorbents possess CEC for both cesium and strontium at 400 cmol(+)/kg1 (Fig. 2). Both are easily made from low-cost raw materials, and can be supplied at a price of around 600 yen/kg.
(5) Ripple effects of this research and impacts on society
As inorganic polymer-based adsorbents are low-priced, high-functioning adsorbents with excellent durability as described above, it is easy to envisage what applications for them. In the first place, they can be used as a safety guard for covering the bottom aspect and sides of the interim storage facilities now being planned. Currently, zeolite is the main material used in designing such facilities. However, there are serious concerns that the zeolite could break down in a highly alkaline environment such as that caused by leachate from rain water that has passed through highly alkaline waste products such as ash. This in turn could cause the zeolite to lose its adsorbent function and become ineffective as a safeguard. Inorganic polymer-based adsorbents do not break down or lose their adsorbent function when exposed to high levels of alkalinity or acidity, and can easily be manufactured and handled in the massive quantities which are required for this application.
(6) Issues for the future
Since this adsorbent has only been developed recently, although its basic functions have been confirmed in the laboratory, full performance assessments against a variety of real-life effluents have not yet been undertaken. It is planned to deliver samples of the adsorbents for assessment by a third party beginning in January 2015.
NB: Test method: A liquid solution containing cations 0.1mol/L was prepared for each type. The inorganic polymer-based adsorbent was then added at a liquid-to-solid ratio of 500:1. After three hours, the solution was stirred and an adsorbency test performed. After the solids had been collected through filtration and cleaned with purified water, an EDS analysis was done to investigate the post-adsorbency test composition.