
OTA Eisuke, Associate Professor
My area of expertise, “organic synthesis”, is a field of chemistry that focuses on creating organic compounds—materials essential to modern life. These compounds are used in everything from the light-emitting materials in smartphones to pharmaceuticals and agrochemicals, forming the foundation of many industries and daily necessities. To create organic compounds, we typically build molecules by forming bonds between atoms, much like assembling structures with LEGO blocks. However, simply forming bonds is not enough. To synthesize complex molecules as desired, we also need methods to selectively ”break” certain bonds after they’ve been formed. Among the various reactive species that can be generated through bond cleavage—such as cations, anions, or carbenes—I focus on cleavage processes that generate radicals, which are short-lived, highly reactive intermediates with unpaired electrons. These radicals are useful in forming new bonds and exhibit unique reactivity that differs from anions and cations. My current research aims to expand the toolbox for generating radicals via bond cleavage. While this is still fundamental research without immediate practical application, I find great excitement in the possibility that it may one day rewrite textbook knowledge.
Breaking chemical bonds is a type of decomposition reaction. Since we often spend a lot of effort building up molecules, we must be very precise when breaking bonds to avoid destroying the entire structure. In an ideal world, we might imagine having a tool that could selectively cut a specific bond—like something out of science fiction. However, molecules are far too small and dynamic in solution for such targeted manipulation to be possible with current technology. As a workaround, chemists have often attempted to weaken specific bonds by modifying the surrounding molecular structure—a strategy that is effective but adds complexity and extra steps to the synthetic process. To streamline this process, researchers have been actively developing techniques to directly cleave bonds that were previously considered unreactive. Photo-induced reactions have drawn attention, as light provides more energy than heat, enabling the cleavage of otherwise unbreakable bonds. The availability of inexpensive light sources, such as LEDs, has also helped advance this field. Over the past 15 years, many new techniques have emerged, and we are starting to see patterns or “playbooks” for how to perform bond cleavage using light. By establishing new methods that allow bond cleavage in unconventional positions, beyond these known patterns, we could unlock new possibilities in molecular synthesis. This could lead to the creation of entirely new molecules or the simplified synthesis of compounds that were once beyond the reach of conventional synthetic approaches.
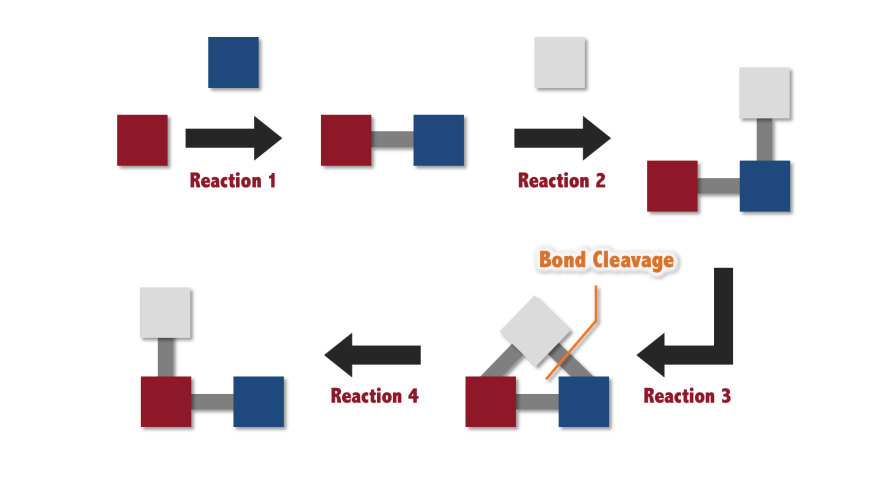
Figure 1. Schematic overview of molecular synthesis. Molecules are synthesized by forming bonds between atoms. However, to achieve flexible and precise molecular synthesis, it is essential not only to form bonds but also to develop methods for selectively cleaving them.
Bond cleavage focusing on interaction of zirconocene with heteroatoms
To develop new patterns of photo-induced bond cleavage, I focused on a catalyst called zirconocene, which contains the metal zirconium (Zr). During my research abroad at Princeton University, I worked on a completely different catalytic system to break hydrogen–oxygen bonds—a groundbreaking method that achieved bond cleavage previously considered difficult. However, it was limited to bonds involving hydrogen atoms. As I explored ways to tackle other types of bond cleavage, zirconium emerged as a promising candidate. Unlike hydrogen or carbon, zirconium strongly interacts with heteroatoms—atoms other than hydrogen and carbon, such as oxygen, nitrogen, or halogens. I hypothesized that this property could be harnessed to break a variety of heteroatom-containing bonds. To use zirconocene catalysts for bond cleavage—especially homolytic cleavage (breaking a bond evenly to form radicals)—they must be converted into an “active” state by donating a single electron. This process, which involves changing the oxidation state of the metal, is known as single-electron reduction. Traditionally, such reduction requires powerful reagents (called reducing agents), but these are so reactive that they can destroy the molecule before the catalyst even becomes active. To avoid this problem, I turned to photocatalysts—substances that convert light energy into chemical energy.
Photocatalysts absorb visible light and enter an excited state, where they can promote single-electron reduction. In practice, we add a photocatalyst that absorbs visible light to a flask, then irradiate the solution with light. The molecules themselves don’t react with the light directly—only the photocatalyst gets excited, which then activates the zirconocene via reduction. This replacement of chemical reductants with photo-induced activation is the core idea of my research.
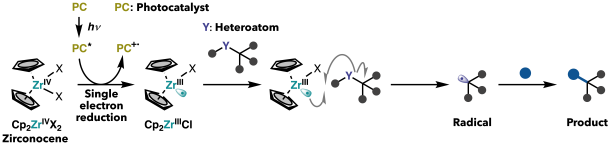
Figure 2. Zirconocene catalyst system for bond cleavage. A photocatalyst excited by visible light activates (reduces) zirconocene, which in turn cleaves bonds in heteroatom-containing molecules to generate radicals.
Using the zirconocene catalytic system I developed, we successfully cleaved various strong bonds—including carbon–oxygen, carbon–chlorine, and even carbon–fluorine bonds. Furthermore, the resulting radical species could be harnessed to form new chemical bonds. Many naturally abundant organic compounds, which often serve as seeds for pharmaceuticals and agrochemicals, contain highly stable bonds. If we can selectively cleave these robust bonds and remodel their structures, we may be able to streamline the synthesis processes for these valuable compounds. Zirconium, as a metal, is relatively inexpensive, and with the widespread availability of LEDs, visible light is now an accessible and practical energy source. Now that this catalytic system is in place, and with zirconium being affordable and LEDs widely available, we’re in a strong position to apply it to a broad range of synthetic challenges. Moving forward, I aim to expand the utility of this zirconocene/photoredox catalysis —not only to break strong and previously unreactive bonds but also to discover entirely new patterns of bond cleavage. In addition to developing new methodologies, I am also applying them directly to molecular synthesis projects that aim to create useful compounds. Ultimately, I hope to demonstrate how strategic bond cleavage can significantly simplify and advance the process of molecular construction.
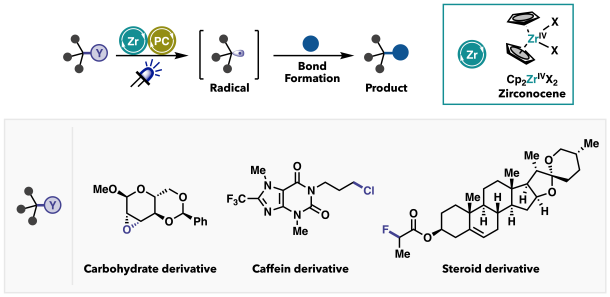
Figure 3. Bond cleavage using the zirconocene/photoredox catalysis. This catalytic system was successfully applied to derivatives of natural products, enabling selective bond cleavage in complex molecules.










