
NISHIDA Nao, Assistant Professor
Studying cancer which is the leading cause of death among Japanese people
I am a cancer researcher. Cancer occurs when the genes of normal cells mutate to grow and multiply infinitely, thereby impairing the functions of normal organs to be detrimental to human health. Cancer not only grows where it has developed, but it can also spread to other organs and continue to grow there as well. This phenomenon is called metastasis. For example, breast cancer is known to spread to the lungs, brain, or liver. Cancer that deprives the functions of the vital organs for survival accounts for the leading cause of death in many countries in recent years; in Japan, one in two people will be diagnosed with cancer in their lifetime.
When a mass of cancerous cells grows, it is called a tumor. However, the tumor lumps are not made up of cancer cells only (See Fig. 1). Approximately up to half of the volume of tumor accounts for immune cells, blood vessels, fibroblasts, and the extracellular matrix that supports tissue structure.
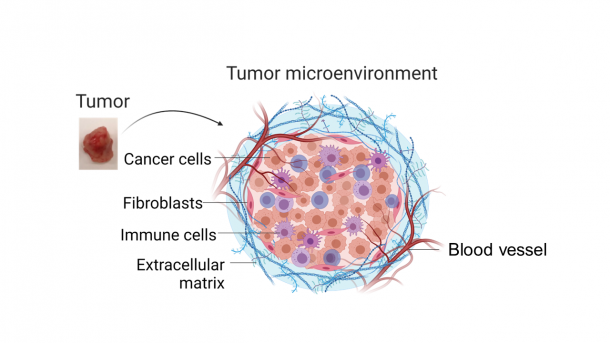
Fig. 1 Structure of tumor. This tissue is composed not only of cancer cells, but also immune cells, blood vessels, fibroblasts, and an extracellular matrix that supports the tissue structure. The figure was created with BioRender.com.
Cancer cells and the surrounding various normal cells exchange information and affect each other within the tissue. In the case of cancer cells, they send a signal that alters the surrounding cells to favor cancer survival. With this signal, for example, immune cells that should normally attack cancer cells are tamed, thereby making it easier for cancer cells to grow and metastasize. In my past experiments, I demonstrated that cancer cells stimulate fibroblasts establishing a feedback loop that stimulates cancer cells to proliferate. Conversely, several reports showed that signals sent by normal tissue environment made cancer cells behave like normal cells while maintaining their genetic mutations. In most cases, the cancer cells overcome those signals from normal cells and modulate the surrounding cells to be on the cancer’s side.
These findings suggest that the nature of the cell highly depends on the environment in which it is located. Therefore, I am currently studying how cells interact within cancer tissue and how the tissue environment affects cancer growth and metastasis.
“Extracellular vesicles”, a mediator of intercellular information transmission
The strategies of exchanging information among cells are categorized into two types: (1) information transmission by direct contact between cells; and (2) communication through information transmitters. I am focusing on “extracellular vesicles” (See Fig. 2), which are one of the information transmitters.
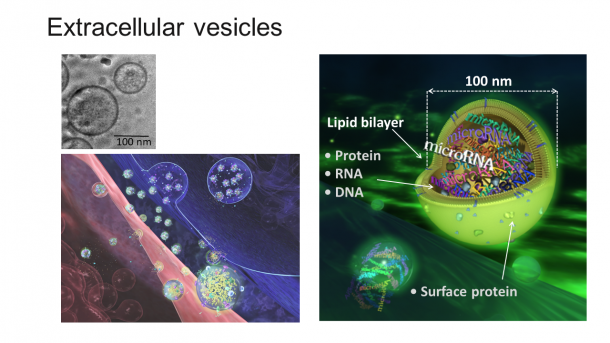
Fig. 2 The extracellular vesicles are spherical structures aligned with lipid bilayer membrane, with a diameter of approx. 100 nm (n is 10-9). They are loaded with proteins, DNA, RNA, and other components that are passed between cells.
Extracellular vesicles are spherical structures aligned with lipid bilayer membrane, with a diameter of approximately 100 nm. They are loaded with components such as proteins, DNA, and RNA. Although extracellular vesicles are secreted by almost all cells, cancer cells are considered to secrete them more than normal cells.
When an extracellular vesicle is released from a cell, it can be taken up by another cell, releasing the internal protein, DNA, RNA, and cause changes in the receiving cell. By passing extracellular vesicles to normal cells, cancer cells alter normal cells to be an ally of the cancer, thereby forming a cancer-specific environment allowing for their proliferation. Furthermore, extracellular vesicles released by cancer cells travel through the bloodstream to disseminate to other organs. The extracellular vesicles precondition the environment of the distal organs so that the cancer easily settles in other organs and forms metastatic lesions. In fact, I demonstrated using a mouse model that antibodies can prevent other cells from uptaking extracellular vesicles released by breast cancer cells, thereby suppressing metastasis to the lungs (See Fig. 3). I used antibodies that bind only to extracellular vesicles derived from cancer cells but not to mouse innate extracellular vesicles present in the blood. The antibodies did not affect the size of the primary tumor.
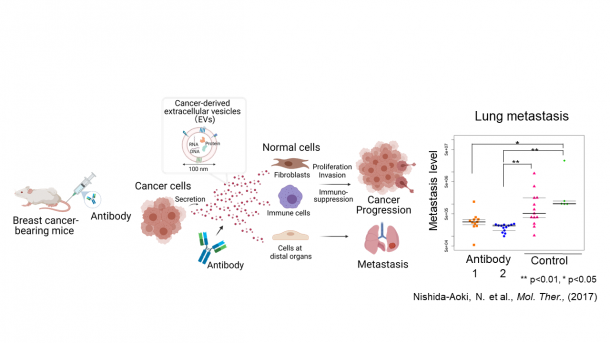
Fig. 3 Antibodies that bind to cancer extracellular vesicles suppressed lung metastasis of breast cancer when administered to mice transplanted with breast cancer cells. The figure was created with BioRender.com.
However, there are many unknowns about the behavior of extracellular vesicles, such as the amount, distribution, distance reached, and speed at which extracellular vesicles released by cancer cells are taken up by which cells in the cancer tissue. One of the reasons is that the extracellular vesicles are extremely small, which makes it challenging to attain detailed microscopic observations within the cancerous tissue structures.
To overcome the experimental limitations, I am utilizing a “tissue slice culture”, which living tissue is thinly sliced and kept alive with a nutrient-containing medium to conduct detailed microscopic observations while maintaining the actual cancer tissue structure. I collected extracellular vesicles from breast cancer cells, stained with a green fluorescent dye called PKH67, supplemented onto the tissue slice, and observed under a fluorescent microscope. I could confirm that the extracellular vesicles were being taken up by the live cells on the tissue slice.
Future Goals
In the future, I would like to use tissue slice cultures to analyze the amount, distance reached, and speed of movement of extracellular vesicles, and which cells have taken up extracellular vesicles into tumor tissue. I also think that there is still room to improve a labeling method for detecting extracellular vesicles.
Although research on the behavior of extracellular vesicles is not well understood because of the many experimental challenges, I believe that the tissue slice culture method can overcome the challenges to understand the extracellular vesicle transfer within tumor tissue.
By understanding the behavior of extracellular vesicles in tumor tissues and revealing the communication between cancer cells and normal cells, we will be able to manipulate cancer-normal cell interactions. I hope to develop new cancer treatments, such as inhibiting the growth of cancer cells and encouraging cancer cells to behave like normal cells.
Coverage/Constitution: AIMONO Keiko
Cooperation: Graduate School of Political Science, Waseda University, J-School










