
- Yoshiyuki Kuroda, Assistant Professor (July, 2014)
The quest for beautiful structures on a nanoscale level
I have been engaged in research to create new functional materials using various host-guest compounds (Fig. 1), including clay minerals. On this occasion, I would like to introduce my research to create new structures using clay minerals. However, the tool I use is not a clay spatula but chemistry. One of the clay minerals that I study has a beautiful structure where geometric patterns are arranged in an orderly manner on an atomic scale (Fig. 1).
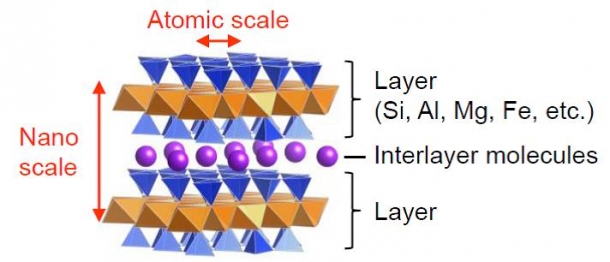
Fig. 1: Structure of a clay mineral. Regular tetrahedrons (blue) and regular octahedrons (brown) primarily containing Si, Al, Mg, and Fe are stacked in layers. These layers are the hosts, and are capable of holding various molecules (purple balls) in between them as the guests. It is easy to understand the structure if you think of the hosts as pieces of sandwich bread and the guests as fillings. The presence of the hosts maintains the geometry, and the guests deliver a variety of tastes (functions).
Some clay minerals have spherical and tubular structures other than the layered structure. (Courtesy of Assistant Professor Yoshiyuki Kuroda)
When looking at the structure in detail, you can see that the groups of regular tetrahedral and octahedral inorganic atoms are arranged systematically to form the layers. Different molecules can be held between the layers. Clay exists abundantly in soil and thus is available at very low prices. With a bit of ingenuity, it is possible to create a highly functional material from a low-cost raw material.
It is very important in my studies to create a substance with beautiful structures using clay minerals. The functions of a clay mineral vary depending on its structure, and therefore controlling the structure is the first step in delivering a new function. This time, I would like to introduce scrolled clay minerals and very small granular clay minerals.
Clay Mineral Nanoscroll ― Peeling Off Coherent Layers One by One
One clay mineral that I used in this study is kaolinite, which has a sheet structure where layers of silica (SiO) and alumina (AlO) seem to be bonded together (Fig. 2: Upper left).
The alumina layer has regular octahedral structures (purple) that are horizontally aligned. The silica layer has regular tetrahedral structures (green) that are horizontally aligned but slightly twisted. In fact, the regular tetrahedrons of the silica layer need to be connected repeatedly at a distance slightly longer than that of the octahedrons of the alumina layer. The tetrahedrons are then forced to be slightly twisted and aligned in a distorted manner when these alumina and silica layers are bonded together, sharing their apexes.
Through the reaction of kaolinite with methanol and a surfactant, the methanol and surfactant enter between the layers so that the layers can be peeled off (Fig. 2). Then, only the silica layers extend to be free of distortion. As a result, the peeled layers roll up (Fig. 2: lower photos).
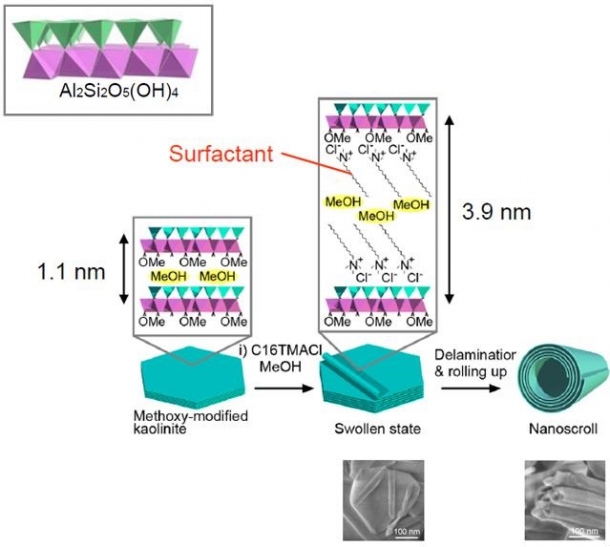
Fig. 2: How to make a clay mineral nanoscroll: A clay mineral nanoscroll can be formed by treating kaolinite with methanol (MeOH) and then mixing it with a surfactant at room temperature. The chemical structure of the surfactant is very important because even one additional piece of carbon changes the efficiency with which the layers are rolled up.
The upper left shows a layer of kaolinite. The purple regular octahedrons and the green regular tetrahedrons present alumina and silica respectively. An aluminum (Al) or silicon (Si) atom is located at the center of each structure and oxygen atoms at the apexes. An oxygen atom is shared by a tetrahedron and an octahedron at their apexes. (Courtesy of Assistant Professor Yoshiyuki Kuroda)
The surface area of the kaolinite nanoscroll is larger than that of the original structure. This increases its performance to adsorb other substances. The performance of the kaolinite nanoscroll in adsorbing contaminants such as dyes is increased up to about four times that of the original kaolinite.
Making Hyperfine Clay Particles – Renewable Anion-Exchange Substance
Some clay minerals with a layered structure can hold anions between the layers. The interaction to hold anions between the layers varies depending on the type of anion. For example, a clay mineral called layered double hydroxide (LDH) holds carbonate ions with a strong interaction and nitrate ions with a weak interaction. The anions held with a weak interaction between the layers are exchanged for anions held with a strong interaction. This is called anion exchange ability. Professor Yamazaki of Waseda University reported that the smaller the LDH particles, the higher this anion exchange ability. It was difficult, however, to reduce the size of the LDH particles and uniformly control them, and there was a challenge in clarifying the relationship between their structure and properties.
I have found a method to easily synthesize hyperfine LDH (Fig. 3). In this method, it is possible to synthesize an extremely small LDH particle of 10 nm (1 nm is a billion part of 1 m). The LDH particles synthesized in my method are relatively uniform in form and size, and thus can be considered to be easy to use for testing the properties of LDH particles.
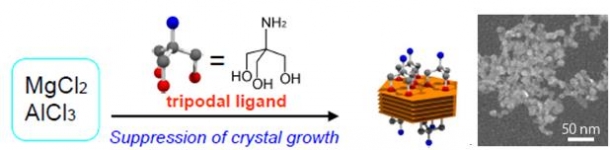
Fig. 3: Method of synthesizing hyperfine LDH particles. LDH particles of 10 nm can be synthesized simply by mixing magnesium chloride (MgCl2) and aluminum chloride (AlCl3) with tripodal ligand and heating the mixture to about 80°C. (Courtesy of Assistant Professor Yoshiyuki Kuroda)
The LDH particles that I synthesized also have a “renewable” aspect. Once carbonate ions strongly held in the interlayer space between the layers, the method of exchanging the ions for other anions is troublesome. This means that once carbonate ions enter into LDH, they cannot be used as the anion-exchange material.
The ultrafine LDH that I synthesized allows carbonate ions to be exchanged for nitrate ions if it is used after treated with a high concentration of sodium nitrate solution. This means that the anion exchange ability can be recovered. The evaluation of clay particles made in a variety of sizes revealed that the anion exchange ability depends on the particle sizes.
I measured the ability to purify contaminated water with the LDH that I synthesized. Water that was contaminated by arsenic (As), selenium (Se), or boron (B) was purified through treatment with hyperfine LDH particles more efficiently than with LDH particles in larger sizes. The treated water meets the drinking water standard (for arsenic and selenium) defined by WHO and the waste water standard (for boron) defined by the Ministry of the Environment in Japan.
This time, I synthesized clays with a high anion exchange ability from low-cost materials such as magnesium and aluminum. I expect that it might be possible to apply this method to battery materials if I used metals such as manganese, nickel, or cobalt.
Results That Defy My Expectations Are Fun for Me
When thinking about a new research theme, I make a prediction that using this method will work fine based on my experiences and the research results by predecessors.
For hyperfine LDH particles, I came up with an idea to combine the layered clay minerals handled in the Kuroda Laboratory of Waseda University (where I first belonged) with a technique of complex chemistry handled in the Mizuno Laboratory of the University of Tokyo, where I belonged as a postdoctoral fellow. Actually, my initial purpose was to peel off the layers of LDH one by one.
It is difficult to do this because carbonate ions entering between the layers bond the layers together strongly. I wondered if, before carbonate ions are tucked into the layers of LDH, attaching ligands onto the layer surfaces like complexes could prevent the carbonate ions from adhering to the layers, so that the layers could be peeled off one by one.
In the experiment, I failed to peel off the layers one by one but succeeded in making them thinner. Then, I increased the concentration of ligands and obtained extremely small granular LDH, defying my expectations. The LDH had a property that allows carbonate ions to be readily exchanged for other anions, which I thought could not be exchanged. The most fun part of research is getting results that defy my expectations.
I would like to continue to synthesize inorganic substances with beautiful structures to create new, highly functional materials. I will also try a theme of creating organic-inorganic hybrid substances with new performance by replacing the ligands to be attached to clay minerals with various organic substances.
Interview and composition: Kaori Oishi
In cooperation with: Waseda University Graduate School of Political Science J-School










