
HOSHINA Naosuke, Assistant Professor
Complex neural circuits for various brain functions
In the brain, there are many neural circuits that allow organisms to perceive and act. These neural circuits are composed of various types of neurons, and these neurons are connected through synapses which allow for the passing of information. However, the specific patterns in the connection and organization of neurons remains to be unclear. To solve this mystery, I specialize in neuroscience, a field in which we observe the inner workings of the brain, and physiological outcomes to evaluate possible functions within neural circuits.
There are approximately 100 billion neurons, and these neurons create nearly 100 trillion synapses. (Fig. 1).
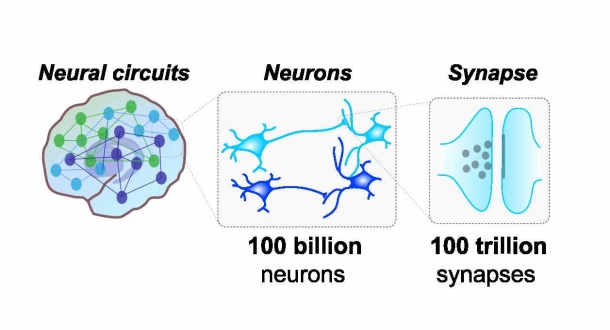
Fig. 1. Schematic View of Neural Circuits in the Brain
Synapses allows for neurons to connect and form complex neural circuits. A synapse transfers information through synaptic vesicles, which are represented as dark circles in the synapse.
Neurons transmit information by releasing neurotransmitters at the synapse. However, there exists a gap between pre- and post-synaptic sites. These gaps are bridged by cell adhesion proteins. Importantly, these synaptic adhesion molecules factor into the synaptic binding between specific types of neurons and serve as “markers” to distinguish complex neural circuits. Furthermore, anomalies relating to neural circuits responsible for specific brain functions may be linked to certain symptoms in psychiatric and neurological diseases.
Therefore, together with several researchers, I have focused on a group of synaptic adhesion proteins referred to as protocadherins (PCDH). Specifically, I have conducted research on PCDH10, PCDH17 and PCDH19 proteins through genetic and neurological analyses to assess the functional role of these synaptic adhesion molecules, and their association with neuropsychiatric disorders.
PCDH17, a synaptic adhesion molecule for parallel cortico-basal ganglia circuits
Previous research indicated that neural circuits connecting the cerebral cortex and the basal ganglia are responsible for sensory and motor functions, executive functions, and emotion control in parallel. However, the specific synaptic adhesion molecule which coordinates the parallel cortico-basal ganglia circuits was unidentified.
To solve this mystery, I have generated an antibody that specifically recognizes PCDH17 proteins and used it to highlight regions with high PCDH17 distribution in mouse brains. As a result, I discovered that PCDH17 is highly distributed only in specific regions of the striatum, globus pallidus, and substantia nigra in the basal ganglia. I also found PCDH17 is highly distributed in the medial prefrontal cortex, and the regions where PCDH17 is distributed are selectively connected (Fig. 2). Therefore, I can speculate that PCDH17 is one of the synaptic adhesion molecules that determines the specificity of “parallel neural circuits.” PCDH17 was the first molecule to show region-specific expression in such parallel circuits and serve as a “marker” for neural circuits whose regions are selectively connected. This shows that the expression pattern of molecules can classify neural circuits and the discovery presents a new perspective in analyzing structures and or functions of neural circuits at a molecular level.
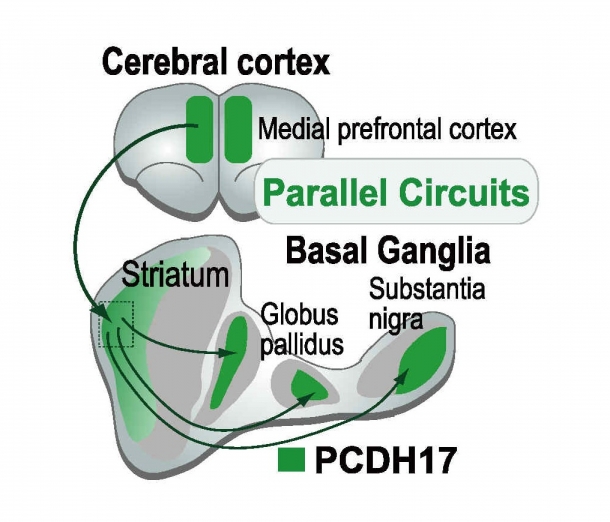
Fig. 2. PCDH17 Distribution of in Mouse Brains
PCDH17 is specifically distributed in some regions of cerebral cortex, striatum, globus pallidus, and substantia nigra in the parallel cortico-basal ganglia circuits.
Furthermore, to understand the role of PCDH17-expressing neural circuits in the brain, I generated the mice deficient in PCDH17. By comparing wild-type and PCDH17 deficient mice, I examined the structures and functions of synapses by using electron microscope analysis and electrophysiological analysis and investigated brain functions through behavioral analysis. As a result, I discovered that the PCDH17 deficient mice had an abnormal increase in accumulation of synaptic vesicles, a change in neurotransmission efficiency, and an abnormality in behavior related to depression. Therefore, PCDH17 acts as a synaptic adhesion molecule for parallel cortico-basal ganglia circuits, and it is involved in depression regulation.
The disorder that occur only in women due to PCDH19 mutations
PCDH19 disorder, which is caused by a mutation in the X chromosome gene PCDH19 (human gene), is an inherited neurological disease that occurs only in women. Symptoms include epilepsy and intellectual disability. However, the reason why it occurs only in women remained unknown. Therefore, I have conducted research to elucidate the mechanism of female specific symptoms.
I examined the structures and functions of synapses by using electron microscopic analysis and electrophysiological analysis between male, female mice with PCDH19 (hereinafter Pcdh19 (mouse genes) wild-type), male mice without PCDH19 (hereinafter Pcdh19 hemi-mutant), and female mice with mixed cells with and without PCDH19 (hereinafter Pcdh19 hetero-mutant). In addition, I evaluated the cognitive functions of these mice through behavioral analysis. As a result, the female Pcdh19 hetero-mutant was found to cause synaptic dysfunction and associated cognitive impairment, while the male Pcdh19 hetero-mutant was normal. As a result, I discovered that female Pcdh19 hetero-mutant mice cause synaptic dysfunction and associated cognitive impairment, while male Pcdh19 hemi-mutant mice had normal synaptic and cognitive functions.
In order to explain the abnormalities in female Pcdh19 hetero-mutant mice, I conducted analyses focusing on the functions of PCDH19 and N-cadherin as synaptic adhesion molecules. In Pcdh19 wild-type and the male Pcdh19 hemi-mutant mice, normal synaptic function is achieved by “match” between synaptic adhesion molecules. However, in the female Pcdh19 hetero-mutant mice, synaptic dysfunction is caused by “mismatch” due to a lack of PCDH19 on one side of the synapse (Fig. 3). This mismatch results in reduction of synaptic transmission efficiency, and even cognitive dysfunction. Through this research I have clarified the causes of inherited neurological diseases that occur only in women and provided a possible treatment strategy for the disorder.
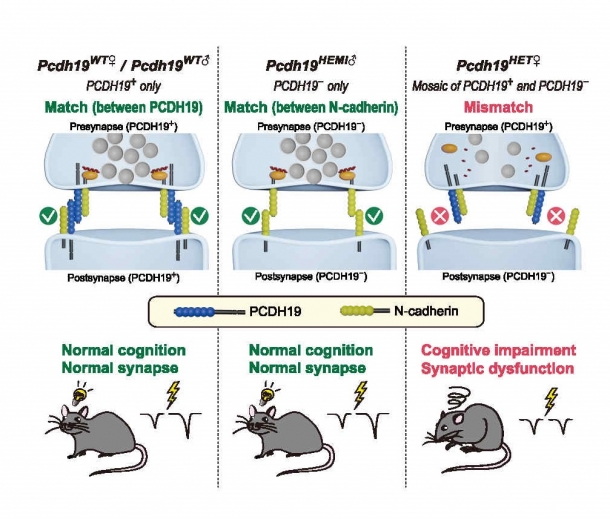
Fig. 3. Mismatch underlies female specific PCDH19 disorder.
At Pcdh19 wild-type synapses, “match” between PCDH19 organizes synapses. At male Pcdh19 hemi-mutant synapses, “match” “match” between N-cadherin organizes synapses. However, at female Pcdh19 hetero-mutant synapses between PCDH19-positive and PCDH19-negative neurons, mismatching of synaptic adhesion molecules leads to synaptic dysfunction. As a result, only the female of the Pcdh19 hetero-mutant mice showed cognitive impairment. Source: Hoshina et al., Science 372, eaaz 3893 (2021)
Role of neuropsychiatric disease-linked molecule PCDH10 in the brain
PCDH10 has been reported to be associated with autism spectrum disorder and obsessive-compulsive disorder in humans. To evaluate functional roles of PCDH10 in the brain, I generated and analyzed mice that lack PCDH10 molecules. As a result, I have found that PCDH10 deficient mice showed abnormal synaptic development in the amygdala, a region of the brain which dictates emotions. Lack of PCDH10 also showed behaviors with decreased levels of anxiety and fear. Therefore, it can be inferred that PCDH10 is responsible for emotional control, such as anxiety or fear, which may be related to neurological disorders such as autism.
Comprehensive analysis of synaptic adhesion molecules for elucidation of brain networks
I have studied the functional roles of PCDH17, PCDH19, and PCDH10 molecules, as well as their association with specific neuropsychiatric disorders and published the results. In the future, I plan to develop my research to elucidate the structures and function of large-scale brain networks by continuing research on the aforementioned PCDHs. Based on new discoveries made with this research, my goal is to contribute to the understanding of the pathophysiology of psychiatric and neurological diseases.
Coverage/Constitution: AIMONO Keiko
Cooperation: Graduate School of Political Science, Waseda University, J-School








