Anode Catalysts for Hydrogen Production from Ammonia
Hydrogen fuel is considered a green, carbon dioxide-free energy source as it can be produced via electrolysis of water using electricity generated from renewable sources. Nevertheless, the storage and transportation of hydrogen fuel is energy-intensive as liquifying hydrogen for storage requires very low temperatures.
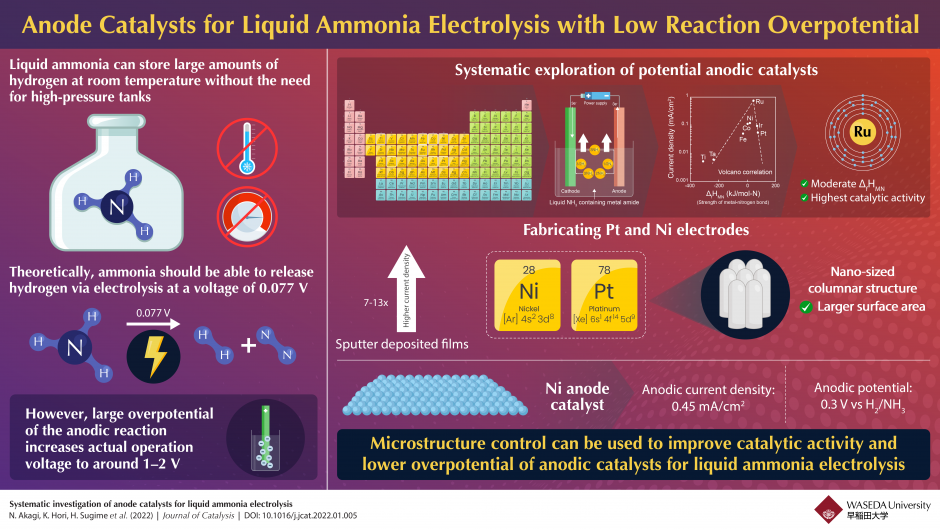
A solution to this problem is using liquified ammonia as the hydrogen source. Ammonia can be liquified at room temperature, does not require high-pressure tanks, and has a theoretical electrolysis voltage of 0.007 Volts (V) for generating hydrogen. In reality, however, the operational voltage can go up to 1 to 2 V due to the large overpotential of anodic reactions.
In a recent study published in Journal of Catalysis, Associate Professor Nobuko Hanada from Waseda University and her team embarked on a mission to find a suitable anodic catalyst that would make the generation of hydrogen from liquid ammonia more efficient. They systematically investigated Pt, Ir, Ru, Ni, Co, Fe, Ta, and Ti for their potential as anodic catalysts.
To test the catalytic properties, the team prepared electrode plates out of the eight chosen metals. They discovered a volcanic-type correlation between current density and metal nitride formation enthalpy, which implied that the catalytic activity of the anodic reaction can be affected by the strength of the metal-nitrogen bond. Ruthenium (Ru) emerged as the metal with the highest catalytic activity due to its moderate affinity to nitrogen, which is beneficial for the decomposition of ammonia to hydrogen via electrolysis.
To test the effect of surface structure on catalytic activity, the researchers designed Ni and Pt films via sputter deposition and tested their catalytic properties.
The sputtered Pt and Ni films showed 7- and 13-times higher current density than the Pt and Ni plates, respectively. A closer look at their surface using electron microscopy revealed a rough surface made up of columnar microstructure. The large surface area due to the presence of microstructures improved catalytic activity and lowered the anodic overpotential. This was especially evident in the case of the Ni film electrode, which achieved a current density of 0.45 mA/cm2 and an anodic potential as low as 0.3 V for ammonia to release hydrogen.
Overall, this research provides a fresh outlook on the improvement of overpotential in anodic catalysts, opening new avenues for increasing the efficiency of hydrogen production from liquid ammonia.
Link to the original journal article: https://linkinghub.elsevier.com/retrieve/pii/S0021951722000057
About the author
Dr. Nobuko Hanada is an Associate Professor at the Faculty of Science and Engineering of the School of Advanced Science and Engineering at Waseda University. She earned her Bachelor’s, Master’s, and PhD degrees from Hiroshima University. As a researcher, Dr. Hanada has an h-index of 24, with over 54 publications which have been cited over 2,900 times. Her work is currently focused on the study of nanomaterials, specifically novel synthesis methods and their application in devices. Her research interests include materials and processes for hydrogen energy utilization, control of kinetics and thermodynamic properties of hydrogen storage materials and development of synthesis processes, as well as design and development of hydrogen storage tanks and hydrogen energy systems based on heat transfer/fluid engineering and process engineering. In addition to that, Dr. Hanada and her team are developing and evaluating technologies aimed at harnessing renewable energy and mitigating global warming.