Discovery of new materials and processes for converting carbon dioxide to carbon monoxide at around 100 °C
The utilization of surplus renewable energy, with minimal heat loss, has become possible
Main points of the announcement
◆ There is a strong anticipation for the reduction of reliance on fossil fuel and the mitigation of carbon dioxide emissions toward the realization of carbon neutrality in 2050.
◆ New materials and processes that enable the chemical conversion of CO2 to CO at temperatures around 100 °C, which conventionally required temperatures ≥ 700 °C, have been developed.
◆ This technology involves the recycling of CO2 as necessary, especially when there is surplus renewable energy, while greatly mitigating heat loss.
The research group led by Professor Yasushi Sekine of the Faculty of Science and Engineering, Waseda University, identified new materials and processes that enable the chemical conversion of CO2 to CO *1, which conventionally required ≥ 700 °C, to be achieved at low temperatures around 100 °C.
There is a high anticipation for the reduction of reliance on fossil fuel and the mitigation of CO2 emissions toward the realization of carbon neutrality in 2050. Under these circumstances, if chemical products can be produced with hydrogen derived from renewable energy, using recovered CO2 as a raw material, it will be possible to recycle CO2 and reduce the reliance on fossil fuels. This technology enables the recycling of CO2 as necessary, especially during the periods of surplus renewable energy, while greatly suppressing heat loss.
The results of this research were published on November 29, 2022 (local time), in the online version of EES Catalysis by the Royal Society of Catalysis.
Article name: Non-conventional low-temperature reverse water–gas shift reaction over highly dispersed Ru catalysts in an electric field
DOI: 10.1039/D2CP04108A
(1) What is known from previous research
Recovered CO2 reacts with hydrogen derived from renewable energy to produce CO in a reaction known as the reverse water-gas shift reaction*2, and this reaction is represented by the following formula.
CO2+H2→CO+H2O
This reaction is endothermic, and hence, it requires a high temperature and has conventionally been conducted as a catalytic reaction at temperatures ≥ 700 °C. The high temperature associated with this reaction poses a challenge, which makes it difficult to drive the process on-demand using renewable energy.
(2) What was newly attempted and clarified in the present research
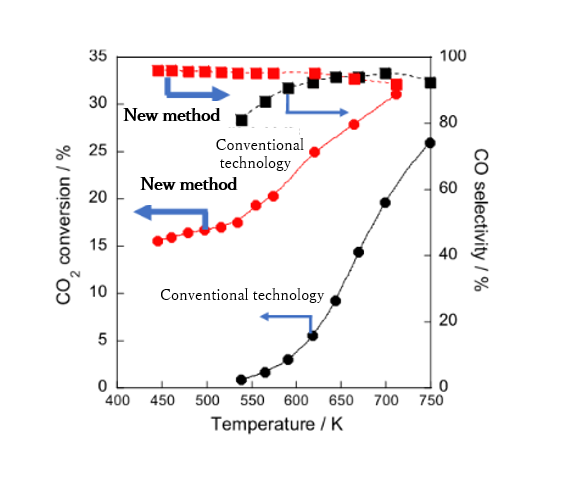
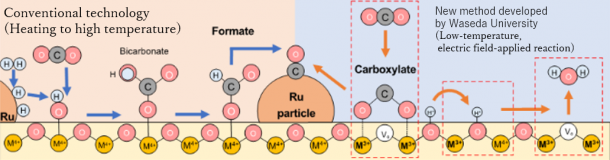
The research group has been investigating a new technology to promote the reverse water-gas shift at a low temperature of around 150 °C, with a high reaction rate and high selectivity. The group developed a catalytic reaction with an externally applied electric field that can achieve this goal and identified catalysts and processes that can exhibit higher performance at low temperatures. The research group found that a heterogeneous catalyst, in which ruthenium metal particles are supported on a stable oxide called zirconium titanate, was very effective for this process.
(3) Newly developed method
The research group found that the application of an external direct current electric field and the induction of surface ionics facilitated the reaction at low temperatures by various operando spectroscopic analysis*3. The reaction was proceeded by a new reaction mechanism, as shown in the figure below.
(4) Ripple effects and social impacts of this research
This technology makes it possible to chemically convert CO2 to CO at a temperature around 100 °C, instead of the conventional temperatures of ≥ 700 °C, and it involves a temperature range close to that of subsequent organic synthesis reactions. Therefore, with this technology, the overall heat loss is greatly reduced, and the process is expected to be driven on-demand. This enables the recycling of CO2 as necessary, especially during the periods of surplus renewable energy.
(5) Future issues
It is expected that the results of laboratory tests at the university will be used as a basis for scaling up the process.
(6) Researcher comments
The reverse water-gas shift is an important reaction with an easily identifiable chemical formula. CO serves as an important building block for various raw chemical materials. However, the reverse water-gas shift reaction requires high temperatures higher than 700 °C. The newly developed technology enables this reaction to easily proceed at a temperature as low as 100 °C, and this has the potential for on-demand chemical synthesis using recovered carbon dioxide. We aim to develop this technology into an exceptional product through collaborations with private companies.
(7) Glossary
*1 Carbon monoxide
A molecule that can be written as CO. It is a very important molecule that could be used as a raw material for the synthesis of various kinds of fuels and chemical products.
*2 Reverse water-gas shift
Reaction of carbon dioxide and hydrogen to produce carbon monoxide. It is an endothermic reaction of approximately 42 kJ/mol; hence, high temperatures are required due to equilibrium constraints.
*3 Operando spectroscopy
This involves live observation of the surface of a solid catalyst in the working environment. It is a technique that allows the viewer to see the nature of a working catalyst.
(8) Article information
・Journal name:EES Catalysis (EES=Energy Environmental Science)
・Article name:Non-conventional low-temperature reverse water–gas shift reaction over highly dispersed Ru catalysts in an electric field
・Author’s name (name of affiliated institution):Ryota Yamano(Waseda University), Shuhei Ogo(Kouchi University), Naoya Nakano(Waseda University), Takuma Higo(Waseda University), Yasushi Sekine*(Waseda University)
・Date of publication (local time):November 29, 2022
・URL:https://pubs.rsc.org/en/content/articlelanding/2023/ey/d2ey00004k
・DOI:10.1039/D2EY00004K
Links
www.f.waseda.jp/ysekine/index_e.html