Discovering that selective capture and desorption of CO2 is possible with a single external potential switch
Main points of the announcement
The high consumption of energy in the selective capture of CO2 is a roadblock to achieving carbon neutrality by 2050.
The research group led by Professor Yasushi Sekine from the Faculty of Science and Engineering, Waseda University, theoretically discovered that selective capture and desorption of CO2 is possible with a single external potential switch by applying an external electrical potential to a solid oxide material with a controlled structure.
Unlike the conventional liquid method, this technology can facilitate dry, compact, and on-demand CO2 capture and concentration processes and emerge as a new technology to help achieve carbon neutrality.
The results of this research were published on October 26, 2022 (local time) in the online version of Physical Chemistry Chemical Physics, published by the Royal Society of Chemistry.
Article name: Theoretical investigation of selective CO2 capture and desorption controlled by the electric field
DOI: 10.1039/D2CP04108A
(1) What is known from previous research
Physical or chemical absorption methods have long been proposed as techniques for CO2 capture. This method involves blowing and absorbing CO2 into a liquid. Although absorption is relatively simple, it is necessary to heat the liquid externally during release, and this consumes ≥10% of the generated electrical power associated with the capture of CO2 from power station exhaust gases.
(2) New achievements and clarifications from this research
If we can practically demonstrate the absorption of the required amount of CO2 into solid material and the desorption and release of the concentrated CO2 using external controls, this innovation will be revolutionary. Based on this concept, the findings of international joint research conducted by Waseda University in Japan and Hanyang University in South Korea have theoretically clarified that selective capture and desorption of CO2 is possible.
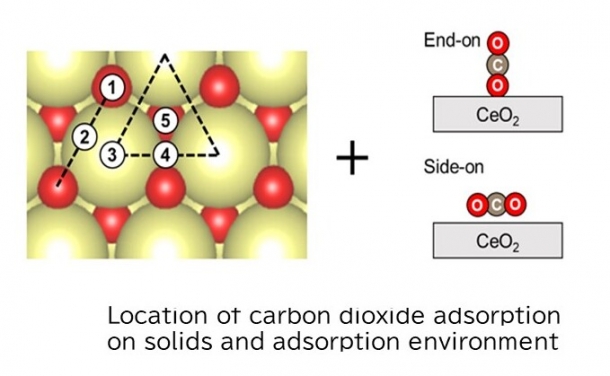
(3) Newly developed techniques to achieve this goal
This study analyzed in detail the absorption adsorption and desorption of CO2 using rare-earth oxides doped*1 with different elements when positive and negative DC potentials were applied externally. The result theoretically clarified that CO2 is selectively sequestered when a strong positive potential is applied (electric current is not applied) and desorbed when this is switched to a negative potential, using a method where dissimilar metals are introduced into a material known as cerium oxide, as shown in the figure.
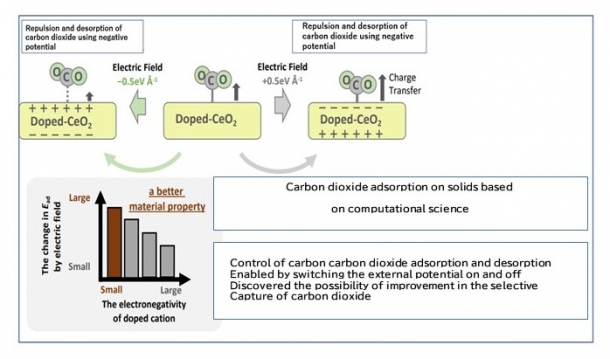
(4)Ripple effects and social impacts of this research
This discovery has shown that, unlike the conventional liquid method, this technology can facilitate dry, compact, and on-demand CO2 capture and concentration processes. If CO2 captured through this method can be recycled or captured in combination with renewable energy, we will take a significant step forward towards attaining a society with reduced consumption of fossil fuels
(5) Future issues
This study was an international joint research conducted during the COVID-19 pandemic which enabled us to theoretically clarify the occurrence of this phenomenon. However, we currently aim to practically demonstrate that CO2 can be sequestered and concentrated with reduced energy expenditure.
(6) Researcher comments
The cost required for CO2 capture ranges from JPY 2000 to 5000 per ton of the exhaust gas, and at least JPY 20000 per ton of the CO2 recovered from the atmosphere. Furthermore, the CO2 capture device used liquid and heat for regeneration, was of considerable size and lacked the ability to navigate sharp turns. If this discovery enables the development of a compact and dry CO2 capture device which can operate on-demand, and efficiently capture and concentrate the required amount of CO2, then it may emerge as a new technology for achieving carbon neutrality.
(7) Glossary
*1 Elemental doping
This method can distort the structure and improve the performance of oxides by substituting trace amounts of impurity elements, which differ from the elements that comprise the oxide structure.
(8) Article information
・ Journal name:Physical Chemistry Chemical Physics
・ Article name:Theoretical investigation of selective CO2 capture and desorption controlled by the electric field
・ Author’s name (name of affiliated institution):Koki Saegusa*1, Kenshin Chishima*1, Hiroshi Sampei*1, Kazuharu Ito*1, Kota Murakami*1, Jeong Gil Seo*2 and Yasushi Sekine*1
*1 Waseda University,Japan
*2 Hanyang University, South Korea
・Date of publication (local time):26 October 2022.
・URL: https://pubs.rsc.org/en/content/articlelanding/2022/CP/D2CP04108A
・DOI:10.1039/D2CP04108A
(9) Research grant
(9) Research grant
Name of research fund: JSPS Bilateral Joint Research
Name of research title: Study on electric field-assisted carbon dioxide sequestration method
Name of research representative (name of affiliated institution): Prof. Jeong Gil Seo (Hanyang University, South Korea)
Links
www.f.waseda.jp/ysekine/index_e.html